Emergency permission to use hydroxychloroquine as a new corona drug revoked

The Emergency Use Permits for Chloroquine and Hydroxychloroquine, which were issued for the treatment of novel coronavirus infection (COVID-19), have been revoked by the
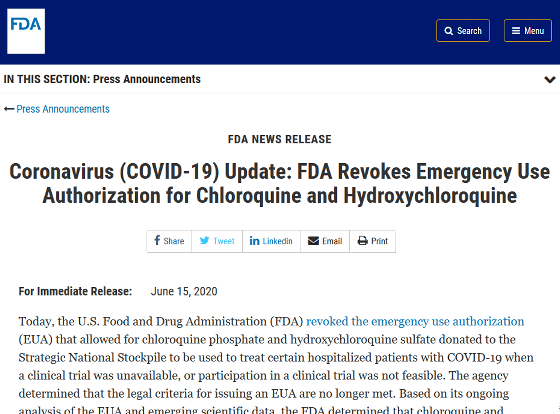
Coronavirus (COVID-19) Update: FDA Revokes Emergency Use Authorization for Chloroquine and Hydroxychloroquine | FDA
https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-chloroquine-and
US FDA pulls its emergency approval of chloroquine use for COVID-19 | Ars Technica
https://arstechnica.com/science/2020/06/us-fda-pulls-its-emergency-approval-of-chloroquine-use-for-covid-19/
FDA revokes emergency use authorization for chloroquine, hydroxychloroquine | Fox News
https://www.foxnews.com/health/fda-revokes-emergency-use-authorization-for-chloroquine-hydroxychloroquine
Hydroxychloroquine, which has been used as an antimalarial drug since the 1950s, has been reported in several studies (PDF file) to improve symptoms of novel coronavirus infections, with the FDA saying that the potential benefits of hydroxychloroquine are , Outweighs the known risks of new coronavirus infections' and was granted an emergency use permit.
Later, hydroxychloroquine once forced the World Health Organization (WHO) clinical trial to be suspended because of the findings that it ' significantly increased the risk of patient death and cardiac complications .' However, due to some doubts about the content of the survey, 'increased risk of death and cardiac complications', the relevant paper was withdrawn and WHO clinical trials have been resumed.
WHO resumes research on hydroxychloroquine as a new corona treatment-GIGAZINE
The FDA's emergency use of hydroxychloroquine was partly due to President Trump's public encouragement to take the drug . However, on Monday, June 15, 2020, Gary Disbro , who is acting representative of the US Agency for Advanced Biomedical Research (BARDA) , requested the FDA to revoke the emergency use permit of hydroxychloroquine. The FDA immediately agreed. 'The withdrawal request was based on information, including the results of the latest clinical trial data, and BARDA concludes that chloroquine and hydroxychloroquine may not be effective in treating a novel coronavirus infection,' the FDA said. I am.
Became the decisive emergency permission to use the cancellation of hydroxychloroquine, of the United Kingdom that are doing a large-scale clinical trials of the new coronavirus infection treatment RECOVERY According to the clinical trials it was. The RECOVERY trial compared more than 1500 patients who received hydroxychloroquine with more than 3000 patients who received standard treatment without hydroxychloroquine. Although not statistically significant, some reported higher mortality in patients receiving hydroxychloroquine. In addition, the known side effects of hydroxychloroquine are also problematic.
The FDA said, 'Hydroxychloroquine should only be used in clinical trials and hospitals, and only where care is available to immediately identify and treat problems caused by side effects of the drug. The known and potential benefits of hydroxychloroquine no longer outweigh the known and potential risks in its permitted range of use.'
Related Posts:
in Posted by darkhorse_log