Oral drug 'Olmient' is approved as a treatment for alopecia areata
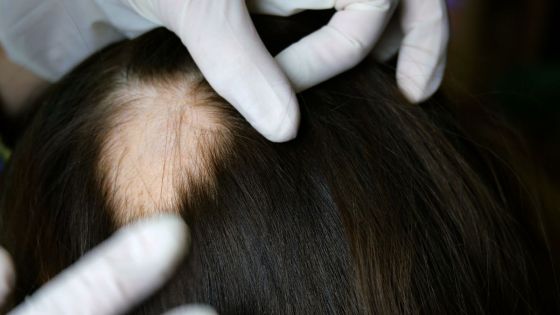
The US Food and Drug Administration (FDA) has approved the oral tablet 'baricitinib (olmient)' used as a treatment for rheumatoid arthritis as an oral treatment for alopecia areata. This is the first time the FDA has approved a drug that contributes to the systemic treatment of alopecia areata.
FDA Approves First Systemic Treatment for Alopecia Areata | FDA
https://www.fda.gov/news-events/press-announcements/fda-approves-first-systemic-treatment-alopecia-areata
The FDA approved Olmient for the treatment of alopecia areata on June 13, 2022. Olmient is used as a therapeutic drug for rheumatoid arthritis, which is thought to be caused by immune disorders, and is said to have the effect of suppressing the progression of rheumatoid arthritis by inhibiting the transmission of inflammatory cytokines . Alopecia areata is also thought to be caused by attacking its own hair follicles due to immune abnormalities, similar to rheumatoid arthritis, and it was expected that the progression could be suppressed by olmient.
Two experiments ( AA-1 and AA-2 ) were conducted in which patients with alopecia areata were given 2 mg or 4 mg of ormient daily for 36 weeks to demonstrate the efficacy of alopecia areata as a treatment for alopecia areata. In Experiment AA-1, 22% of 184 patients who received 2 mg of Olmient per day and 35% of 281 patients who received 4 mg succeeded in suppressing the progression of alopecia areata. Furthermore, in Experiment AA-2, 17% of 156 patients and 32% of 234 patients who received 2 mg of Olmient per day succeeded in suppressing the progression of alopecia areata.
Kendal Marcus, director of dermatology and dentistry at the FDA's Center for Drug Evaluation and Research, said that Olmient was approved for the treatment of alopecia areata. Helps to meet. Access to safe and effective options is very important for many Americans suffering from severe alopecia areata. '
It has been reported that taking Olmient may be accompanied by side effects such as upper respiratory tract infection and shingles. The following site published by Eli Lilly, the developer of Olmient, details the effects and side effects of Olmient as a treatment for rheumatoid arthritis.
Information site for rheumatoid arthritis treatment
https://www.olumiant-patient.jp/

Related Posts:
in Science, Posted by log1o_hf