A test kit that can diagnose new coronavirus infection in 45 minutes is approved in the United States
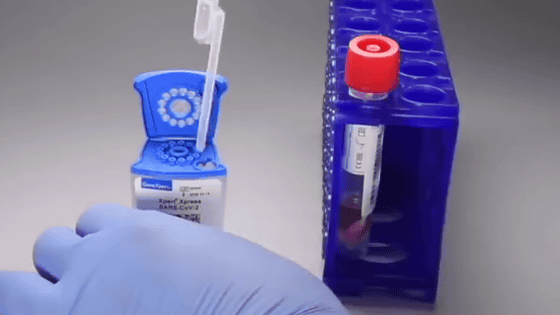
Testing for a new type of coronavirus infection (COVID-19) can take anywhere
Cepheid | Xpert® Xpress SARS-CoV-2 has received FDA Emergency Use Authorization
https://www.cepheid.com/coronavirus
Cepheid's New Covid-19 Test Shows Results in 45 Minutes (1)
https://news.bloomberglaw.com/health-law-and-business/fda-approves-first-bedside-covid-19-test-by-cepheid
FDA grants 'emergency use' coronavirus test that can deliver results in 45 minutes
https://www.cnbc.com/2020/03/21/fda-grants-emergency-use-coronavirus-test-that-can-deliver-results-in-45-minutes.html
It is important to identify and isolate infected individuals early to prevent the spread of new coronavirus infections. However, new coronavirus tests are limited to those performed by health authorities, and there is no test that can be performed by individual hospitals. The tests performed by health authorities have been criticized in the United States for being 'unbearably slow and exacerbating the situation,' as it takes one or several days for results to be obtained.
`` SAR-CoV-2 Xpert Xpress '' developed by Cepheid is a first aid for hospitals and Urgent Care Centers where patients are diagnosed without sending samples to research institutions (first aid for injuries and illnesses that are not life-threatening). Inspection kits that can give results at facilities that specialize in such applications. Since the time-consuming process of sending the sample to the medical institution is cut, testing using 'SAR-CoV-2 Xpert Xpress' will give a diagnostic result in only 45 minutes.
'SAR-CoV-2 Xpert Xpress' uses the 'GeneXpert' device developed by the company for testing HIV and tuberculosis. Cepheid also publishes a movie explaining what kind of test kit 'SAR-CoV-2 Xpert Xpress' is.
Cepheid's Xpert® Xpress SARS-CoV-2 Test (for the Virus that causes COVID-19 & the GeneXpert® System-YouTube
This is the real thing of 'SAR-CoV-2 Xpert Xpress'.

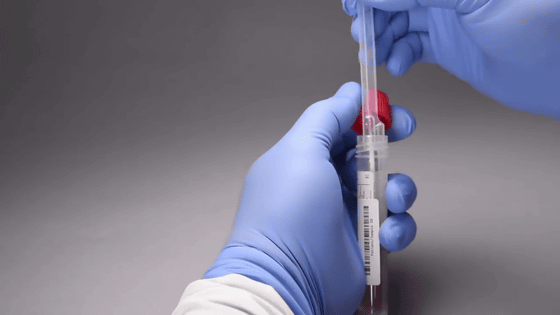
First, obtain a sample from the patient's nasal mucosa.

Insert the sample into the tube containing the test reagent. Shake and mix.

Pipette out the sample and reagent mixture ...
Pour into the 'SAR-CoV-2 Xpert Xpress' package.
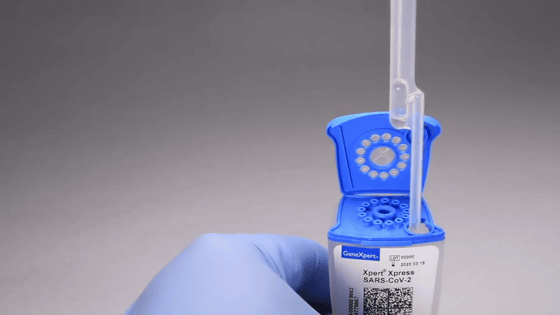
Insert the package into 'GeneXeprt'. Inspection starts automatically.
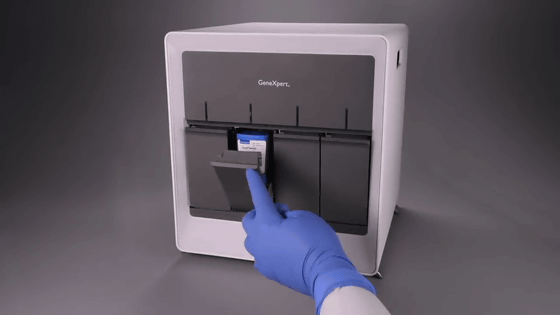
'GeneXeprt' can be operated continuously for 24 hours, but the number of simultaneous inspections differs depending on the model of the equipment.


The largest model can perform 80 simultaneous inspections. It is said that inspection using 'SAR-CoV-2 Xpert Xpress' can be performed without special training.
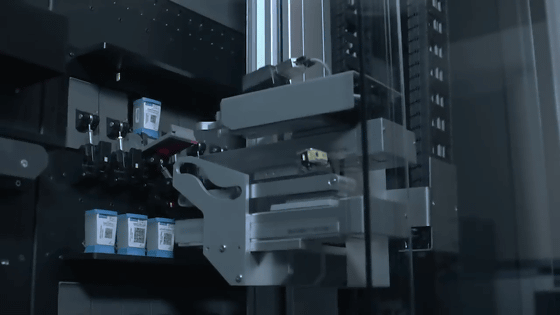
'SAR-CoV-2 Xpert Xpress' has already been approved by the FDA and is scheduled to ship on March 27, 2020. Inspection using 'SAR-CoV-2 Xpert Xpress' is expected to start on March 30.
Cepheid states that 23,000 units of GeneXpert required for `` SAR-CoV-2 Xpert Xpress '' are sold worldwide, of which 5000 units exist in the United States, and `` SAR-CoV-2 Xpert Xpress '' He explained that there are many medical institutions in the United States that can perform the tests used immediately. 'In this era of increasing demand for hospital services, it is revolutionary to be able to perform accurate tests in the vicinity of patients during this period of growing demand for hospital services. This product is a new Corona product,' said David Persing, Chief Medical Technology Officer at Cepheid. It can help ease the pressure on healthcare providers following the crisis of viral infections. '
Related Posts: