Stand-alone new home test kit for new coronavirus infections received first approval from FDA

A standalone standalone test kit for coronavirus infection (COVID-19) that can be used at home, which was being developed by medical-related startup
Coronavirus (COVID-19) Update: FDA Authorizes First Standalone At-Home Sample Collection Kit That Can Be Used With Certain Authorized Tests | FDA
https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-standalone-home-sample-collection-kit-can-be-used
Our commitment to fighting COVID-19-Everlywell: Home Health Testing Made Easy-Results You Can Understand
https://www.everlywell.com/blog/news-and-info/our-commitment-to-fighting-covid-19/
Everlywell gains first FDA authorization for a standalone, at-home, COVID-19 test sample collection kit | TechCrunch
https://techcrunch.com/2020/05/16/everylwell-gains-first-fda-authorization-for-a-standalone-at-home-covid-19-test-sample-collection-kit/
Everlywell is pleased to announce that on May 16, 2020, local time, the FDA's Emergency Use Authorization (EUA: Emergency Use Authorization (EUA) is a test kit 'COVID-19 Test Home Collection Kit' that can be used at home to diagnose a new coronavirus infection. It has been announced that we have obtained (use permission). Everlywell's home test kit will be available in the second half of May 2020.
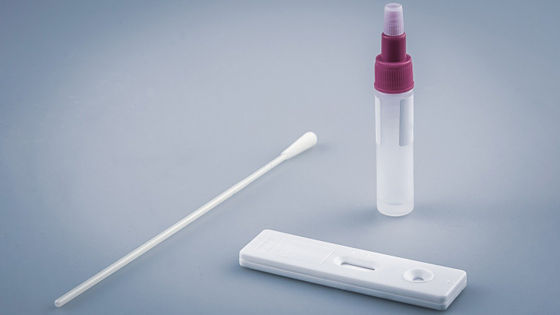
The test method adopted in the 'COVID-19 Test Home Collection Kit' is a nasopharyngeal swab , and it is necessary for the user himself to send a sample taken from the back of the nose with a cotton swab to two laboratories certified by CLIA. However, diagnostic results will be available online within 24-48 hours after the laboratory receives the sample.
In addition to issuing the EUA to Everlywell's home test kit, the FDA has also approved a diagnostic test for new coronavirus infections conducted at two CLIA-accredited laboratories that produce diagnostic results. Has been individually approved by the EUA. ' Two CLIA certified laboratories, Fulgent Therapeutics and Assurance Scientific Laboratories , diagnose the samples collected by the test kit.

Everlywell's home testing kit is the first standalone home diagnostic process issued by the FDA with an EUA. In the case of the diagnostic process using other test kits, it is necessary to have a collaborating organization such as a designated doctor diagnose the sample, but in the case of Everlywell's home testing kit, the sample should be submitted to multiple collaborating laboratories. It is possible to get a diagnosis. Another feature is that all of the cooperating institutions have obtained EAU. In other words, if you use Everlywell's home-based test kit, you can receive 'a diagnostic process that functions independently without relying on an external organization', so it is possible to provide a wider and more stable diagnostic process than previous test kits. I will.
Jeffrey Schlenn, MD, FDA, said, 'The approval of a home-based test kit for a new coronavirus infection that can be used for multiple tests in multiple laboratories will make it easier for patients to undergo tests.' Not only that, but it should also protect non-infected people from potential exposure. '
'It's the first time the FDA's EUA has been issued to a digital medical product manufacturer like Everlywell,' according to Everlywell. Everlywell has not only cooperated with the FDA to obtain an EUA for a home test kit for a new coronavirus infection, but also has a test kit for a new coronavirus infection for clinical use of 30,000 new coronavirus infections. It is said that it has provided about points.
In addition, Everlywell's home test kit 'COVID-19 Test Home Collection Kit' was announced on March 23, 2020. `` As of March 18, 2020, Everlywell plans to expand its infrastructure by collaborating with multiple laboratories in the United States to provide 250,000 test kits each week. '.
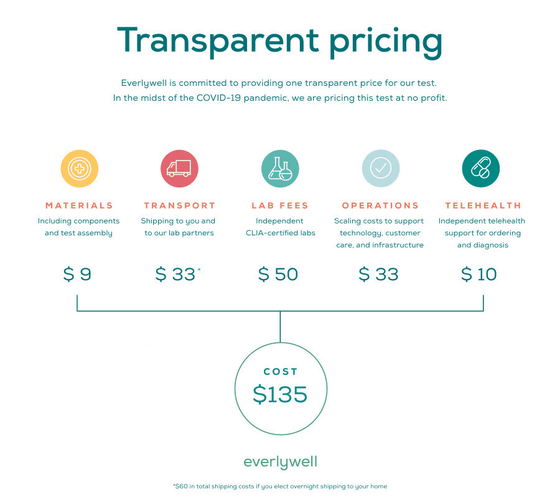
The price of 'COVID-19 Test Home Collection Kit' is $ 135 (about 14,000 yen), but Everlywell says that 'it has no profit for us', so it seems that it will offer an inspection kit at a price different from the cost .. The breakdown of $ 135 is as follows, the material of the test kit is $ 9 (about 1000 yen), the cost of delivering the test kit to the user's home and delivering the collected sample to the laboratory is $ 33 ( (Approximately 3500 yen), a fee of 50 dollars (about 5400 yen) paid to CLIA accredited laboratory for diagnosing samples, the cost of operating a home inspection kit such as technology support, customer care, infrastructure maintenance is $ 33 (about 3500 yen) ), And the telehealth-related cost is 10 dollars (about 1100 yen).
Related Posts:
in Note, Posted by logu_ii