FDA approves nasal spray alternative to EpiPen

The U.S. Food and Drug Administration (FDA) has approved
ARS Pharmaceuticals Receives FDA Approval of neffy® (epinephrine nasal spray), the First and Only Needle-Free Treatment for Type I Allergic Reactions, Including Anaphylaxis - ARS Pharmaceuticals
https://ir.ars-pharma.com/news-releases/news-release-details/ars-pharmaceuticals-receives-fda-approval-neffyr-epinephrine

FDA Approves First Nasal Spray for Treatment of Anaphylaxis | FDA
US FDA approves nasal spray alternative to EpiPen for allergic reactions | Reuters
https://www.reuters.com/business/healthcare-pharmaceuticals/us-fda-approves-first-nasal-spray-allergic-reactions-2024-08-09/
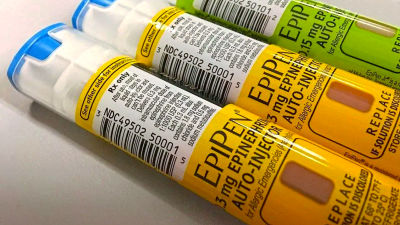
Anaphylaxis is an allergic reaction that causes severe symptoms in the digestive and respiratory tract, and when symptoms appear, an injection of an EpiPen, an injectable medication, is generally required immediately.
Pharmaceutical company ARS Pharmaceuticals has developed a nasal spray called 'Neffy' as an alternative to the EpiPen. Like the EpiPen, Neffy is a product for administering epinephrine, but its unique feature is that it can be administered through the nose instead of by injection. It is expected that the lack of a needle will make it easier to administer epinephrine.
In addition, because it is less bulky than a syringe, it may also reduce the burden on people who have to carry the medication.

A preliminary study of 175 people showed that blood epinephrine levels were comparable between the existing EpiPen and Neffy.
'Some people, particularly children, are afraid of injections, which can lead to delaying or avoiding treatment,' said Kelly Stone, M.D., director of the FDA's Center for Drug Evaluation and Research. 'Today's approval makes this the first epinephrine formulation for the treatment of anaphylaxis that does not need to be administered by injection.'
'This approval marks the first significant innovation in epinephrine delivery in more than 35 years and provides the first and only needle-free treatment option for patients and families experiencing severe allergic reactions,' the FDA said in a press release.
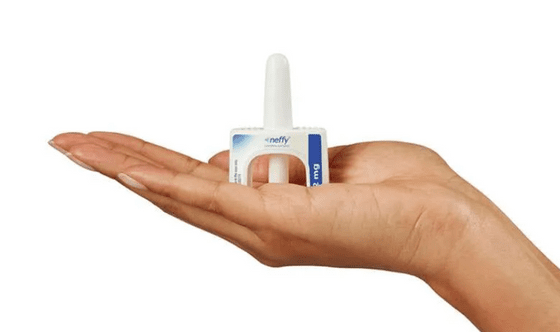
Neffy is expected to be available in the US within eight weeks of FDA approval. It is approved for use in pediatric and adult patients weighing 30kg or more, and patients can purchase two doses for $199.
Following the FDA approval, ARS Pharmaceuticals' stock price rose by more than 13% at one point.
Related Posts:
in Posted by log1p_kr