The FDA approves the generic drug of 'epipen' that suppresses the acute allergic reaction, approval as a generic version for the first time
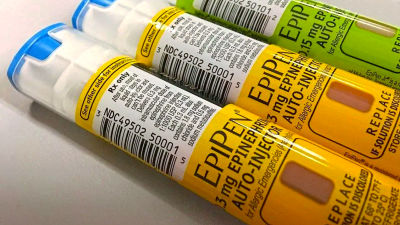
The US Food and Drug Administration (FDA) approved the generic version epipen and automatic syringe developed by Mylan, a pharmaceutical company. It is the first time that epipen generic drugs have been approved.
FDA approves first generic version of EpiPen - The Washington Post
https://www.washingtonpost.com/news/to-your-health/wp/2018/08/16/fda-approves-first-generic-version-of-epipen/
FDA approves first generic EpiPen - ABC News
https://abcnews.go.com/US/fda-approves-generic-epipen/story?id=57221884
Epipen is a medicine that suppresses the acute allergic reaction which develops due to insect bites. People with a high possibility of causing an acute allergic reaction in daily life can be used as a medicine to suppress the deterioration of the symptoms until they are delivered to a medical institution when the episode is developed.
Epipen was trading at a price of about 100 dollars (about 11,000 yen) in 2009, but in August 2016, according to the manufacturer's Teva Pharmaceutical , the price is 600 dollars (about 67,000 yen) It was raised. Naturally, Teva Pharmaceutical was brought to a major criticism as a lifting of an unreasonable price, but this correspondence was never improved, and it was expected that a low-priced version of Epipen appeared.
Regarding the approval of EpiPen's generic drugs, FDA Secretary Scott Gottlieb said: "This approval was one of my longstanding commitments aimed at" allowing for the selection of low-cost alternatives " "I say.
He also said, "It is important to be able to select low-priced options as well as offer stable supply of drugs to those who have severe allergies and who have to prescribe epipens many times." It explains that it corresponds to epipen price rise.

Mr. Gottlieb explained "Generic version epipen was a more challenging approval than general medicine". Epipen approved by the FDA is not only a drug to be injected into the human body but also an automatic syringe that makes it easy to administer even if it is not a doctor. Mr. Gottlieb says "Products with complicated mechanisms are difficult to copy, it takes time to develop similar generic epipens at other pharmaceutical companies," even at other pharmaceutical companies We are planning to work on making similar products efficiently developed.
Related Posts:
in Note, Posted by darkhorse_log