US Food and Drug Administration publishes website comparing performance of new coronavirus test
EUA Authorized Serology Test Performance | FDA
https://www.fda.gov/medical-devices/emergency-situations-medical-devices/eua-authorized-serology-test-performance
One of the widely used tests for COVID-19 is the ' serologic test '. This tests for the presence or absence of antibodies against the novel coronavirus (SARS-CoV-2) contained in blood samples to test whether the subject has been infected with SARS-CoV-2.
by
If it is possible to perform serological tests on a large number of people and determine the prevalence of COVID-19 by examining the number of people who have antibodies, we can grasp the spread of COVID-19 and policies on economic resumption. For reference, the FDA has issued an EUA for several products developed for serological testing.
On the other hand, some serological tests are so unreliable that the CEO of a pharmaceutical company criticizes them as 'disaster levels.'
'The poorness of the new coronavirus antibody test is a disaster level,' said the CEO of a major pharmaceutical company, and what is the problem of the antibody test? -GIGAZINE
In addition, there are issues such as ' false negatives ' in which serological tests result in negative results even though they are infected with COVID-19, and ' false positives ' in which they are diagnosed as positive despite not being infected. Therefore, having a large serological test also increases the risk of this problem.
Therefore, in order to enable medical institutions and specialists to carry out serological tests more accurately, the FDA has published the performance of serological tests collectively based on the information confirmed at the time of EUA examination.
For example, this is the performance of 'Abbott Architect SARS-CoV-2 IgG,' which is an analytical device of the American pharmaceutical company Abbott, which is also used by the Ministry of Health, Labor and Welfare for antibody testing and is an 'Architect' test agent. Since 'Target' is a generic term for the viral genome and proteins that wrap the genome, ' nucleocapsid ' and 'Antibody (antibody)' is ' IgG (immunoglobulin G) ' It can be seen that the test is for the presence or absence of immunoglobulin G, which is an antibody that reacts with the nucleocapsid of SARS-CoV-2.
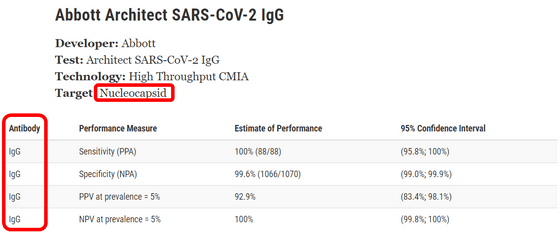
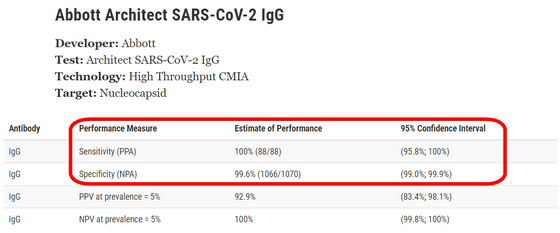
In the test using 'Abbott Architect SARS-CoV-2 IgG', the '
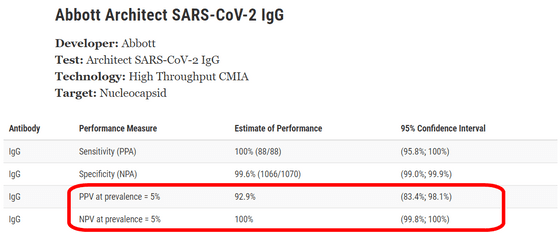
In addition, with a prevalence of 5%, the ' positive predictive value (PPV) ' was 92.9%, with a 95% confidence interval of 83.4-98.1%. Similarly, with a prevalence of 5%, the ' negative predictive value (NPV) ' was 100% and the 95% confidence interval was 99.8-100%. The FDA's published website summarizes similar assessments for multiple serological test methods and allows you to compare the features and performance of each test method.
In the presentation, the FDA said, 'In order to properly carry out serological tests, it is important to understand the performance characteristics and limits of the tests. Whether it will prevent infection or how long the antibody will be effective will be important, but this is still under study.'
Related Posts:
in Science, Posted by log1l_ks