Why is it difficult to compare new corona vaccines?
The new coronavirus infection (COVID-19) vaccine includes
Why you can't compare Covid-19 vaccines --YouTube
In February 2021, the COVID-19 vaccine developed by J & J was approved by the US Food and Drug Administration (FDA). This J & J COVID-19 vaccine is different from the vaccines that have been approved by the FDA so far in that it can be inoculated once.

J & J's COVID-19 vaccine was scheduled to ship 6200 bottles to Detroit, Michigan in early March 2021.

But for this vaccine, 'I don't need it. The Moderna and Pfizer vaccines are the best. I will do everything I can to ensure that Detroit citizens have the best vaccine,' said the Mayor of Detroit.

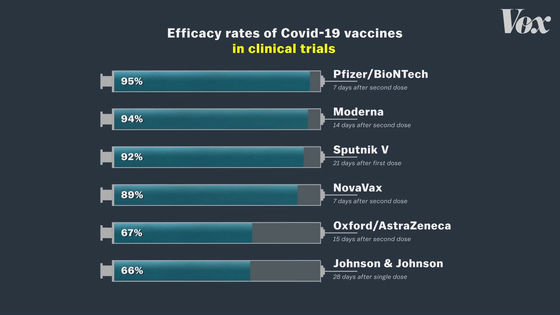
The Mayor of Detroit mentioned that J & J's COVID-19 vaccine is not the best because it is less effective than other vaccines. The Pfizer and Moderna vaccines are characterized by very high efficacy rates of 95% and 94%, respectively.
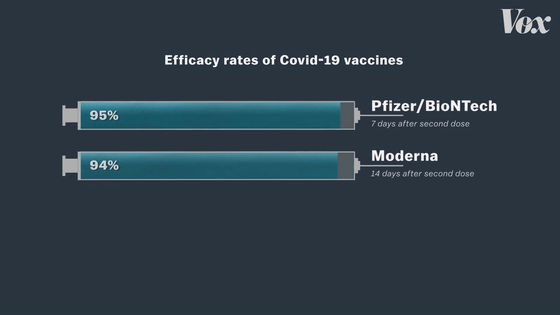
In contrast, J & J's COVID-19 vaccine has an efficacy rate of only 66%.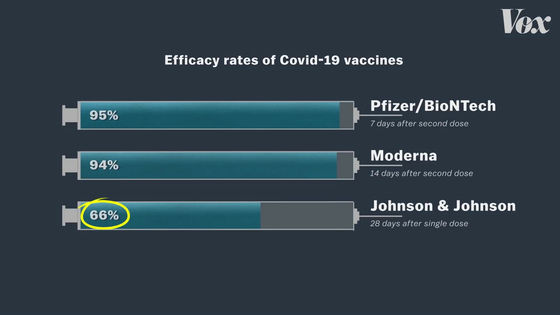
Looking at these numbers alone, J & J's COVID-19 vaccine does not seem to be the best, as the Mayor of Detroit says. But 'that assumption is wrong,' Vox asserts. Regarding vaccine efficacy, Vox said, 'It's definitely not the most important indicator, and it's not even the most important indicator of vaccine efficacy.' To understand why efficacy is not important, we first need to understand how vaccines work.
Vaccine efficacy is a number calculated in large-scale clinical trials with tens of thousands of people.
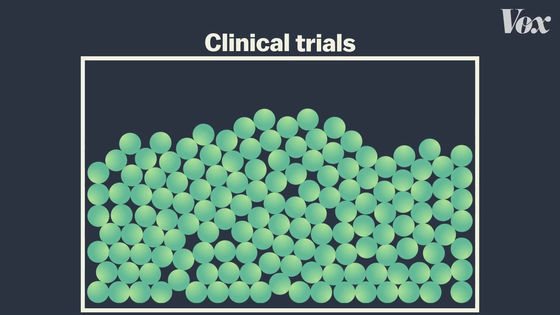
In clinical trials, subjects are divided into two groups. Half are vaccinated and half are vaccinated with a completely ineffective vaccine (placebo).
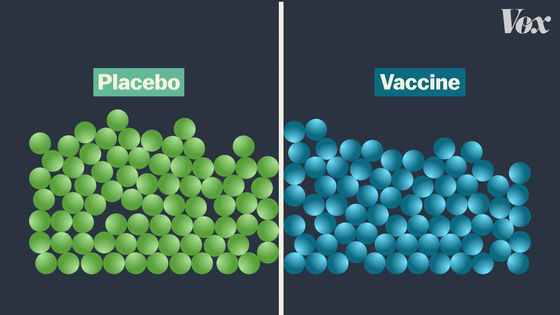
Subjects will then be followed up for several months to see if they have been infected with COVID-19.
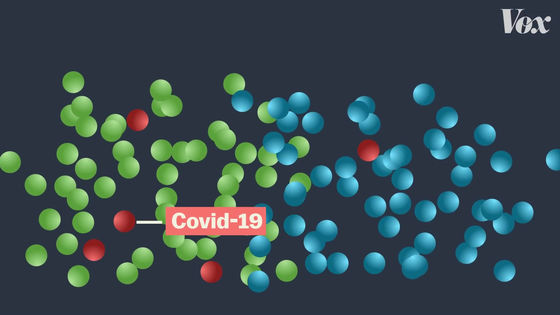
For example, in the case of Pfizer vaccine, 43,000 people are participating in clinical trials ...
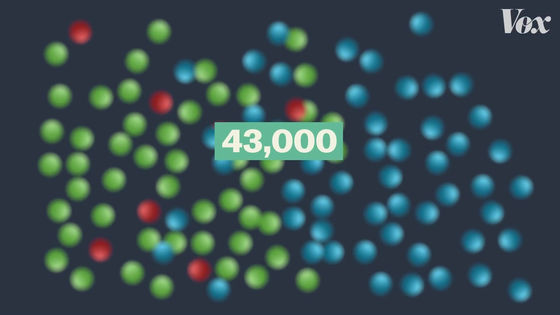
170 people were infected with COVID-19.
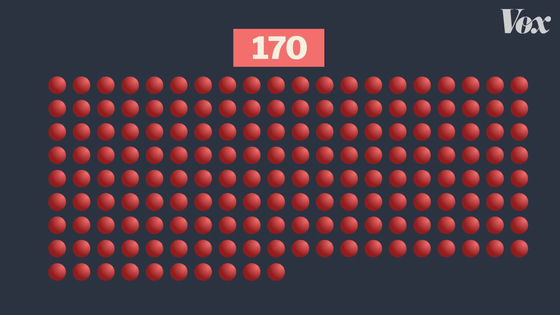
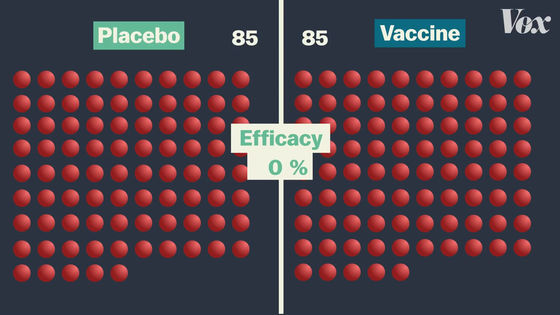
The final vaccine efficacy rate is calculated depending on which group the infected person falls into. For example, if 170 infected people were evenly divided into the vaccine group and the placebo group, the vaccine efficacy rate would be 0% because the rate of COVID-19 infection would not change with or without vaccination. Become.
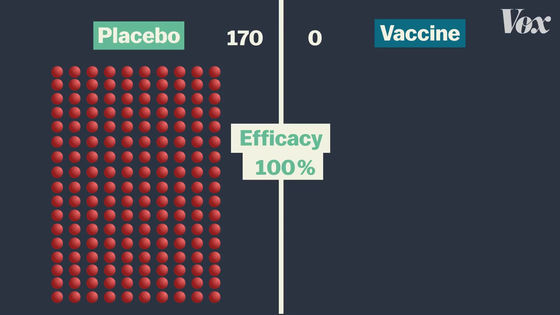
If all 170 people infected with COVID-19 are in the placebo group, then none of the vaccinated people are infected with COVID-19, so the vaccine efficacy rate is 100%.
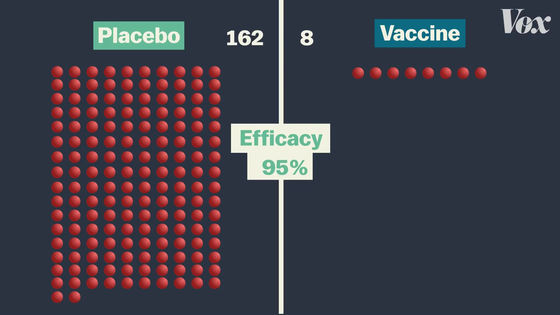
In the Pfizer vaccine clinical trial, there were 162 patients in the placebo group and only 8 patients in the vaccine group, resulting in a vaccine efficacy rate of 95%. This means that you are 95% less likely to get infected with COVID-19.
This does not mean that if 100 people are vaccinated, 5 will be infected.
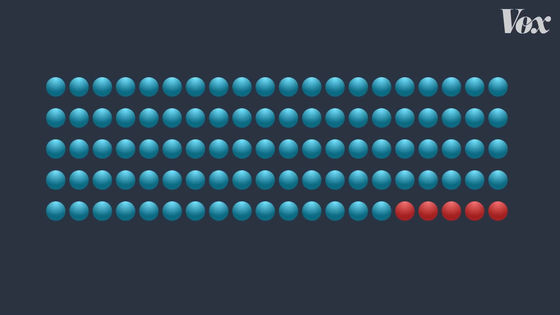
The number 'effective rate 95%' applies to individuals and means that 'when vaccinated, the probability of infection is 95% lower than when not vaccinated'.
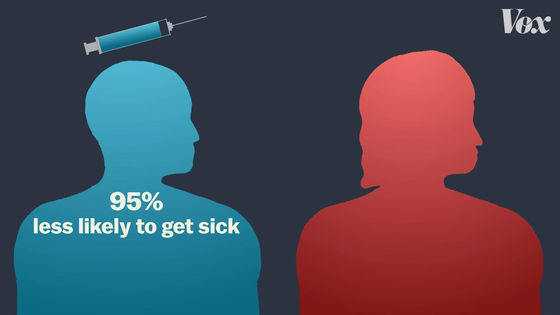
'Every vaccine uses the same formula to calculate efficacy, but clinical trials are conducted in different situations,' said Deborah Fuller, a microbiology expert at the University of Washington. The most important thing to consider when looking at vaccine efficacy is 'when clinical trials were conducted.' '

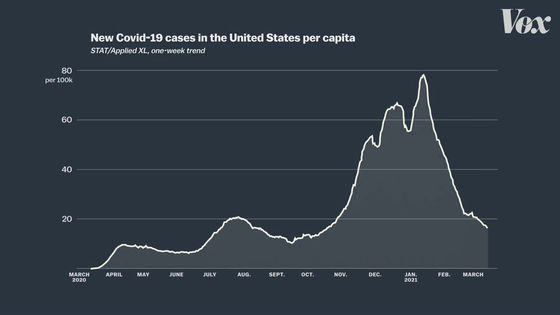
The graph below summarizes the number of new COVID-19 infections in the United States. From November 2020 to January 2021, the number of newly infected people is increasing rapidly.
Clinical trials of the modelna vaccine are conducted entirely in the United States and will be conducted from August to early November 2020.
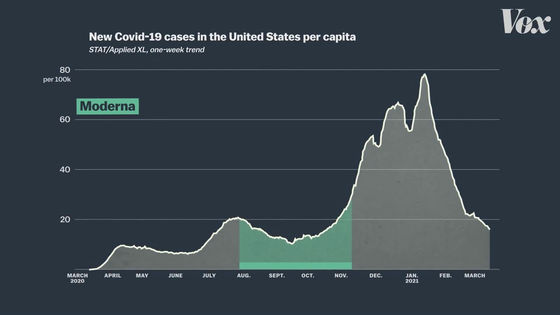
Clinical trials of the Pfizer vaccine are also being conducted mainly in American subjects, and the trial period is also from August to November 2020.
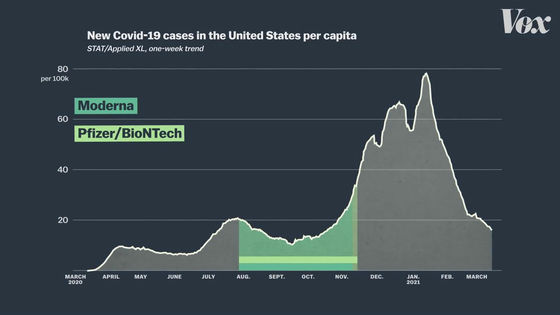
On the other hand, clinical trials of the J & J vaccine are being conducted from November 2020 to January 2021, when the number of newly infected people is higher.
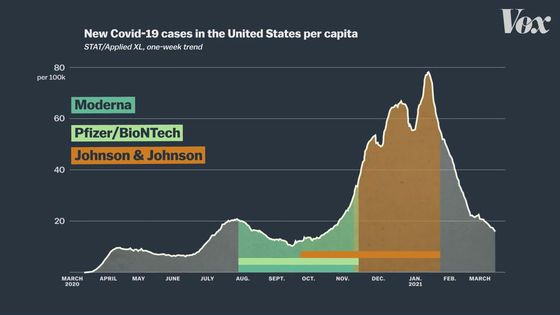
In addition, clinical trials of the J & J vaccine are being conducted not only in the United States, but also in regions such as South Africa and Brazil.
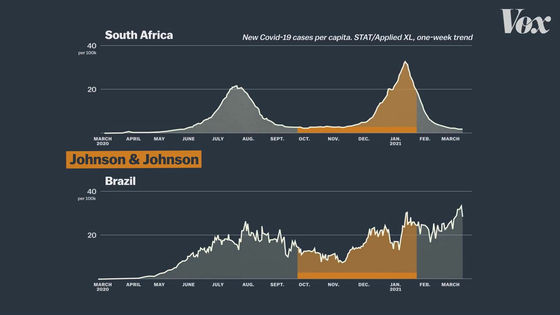
In South Africa and Brazil,
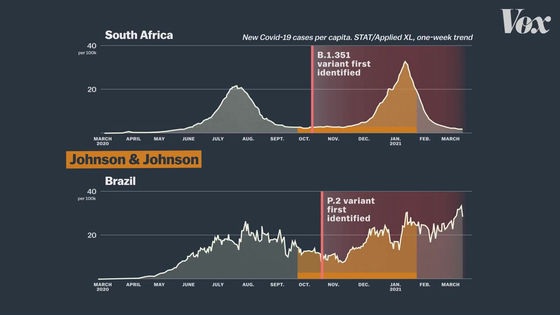
In fact, in clinical trials in South Africa, most cases were infected with mutant strains rather than the usual new coronavirus.
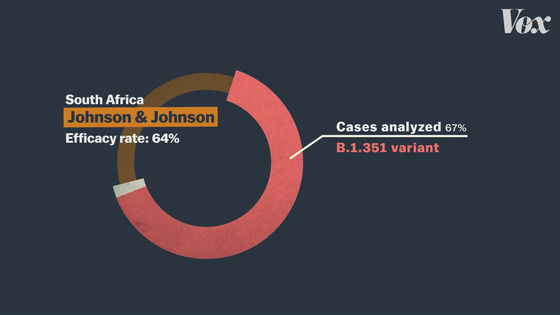
'If you want to compare the effectiveness of vaccines correctly, you need to conduct clinical trials in the same area under the same conditions. If Pfizer and Moderna vaccines are J & J vaccines,' said Amesh Adalja of the Johns Hopkins University Health and Security Center. If tested under the same conditions as, the vaccine efficacy rate would be quite different from current figures. '

In other words, vaccine efficacy only tells you what happened in a clinical trial, not exactly what happens in the real world. As a result, many experts argue that 'effectiveness is not the best indicator for making a correct vaccine decision.'
Also, the purpose of the vaccine is not to prevent infection, but to reduce the number of seriously ill patients and deaths from infectious diseases. In fact, existing COVID-19 vaccine clinical trials have resulted in serious cases and deaths in the placebo group ...
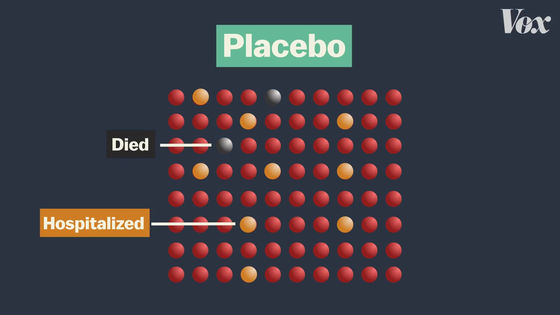
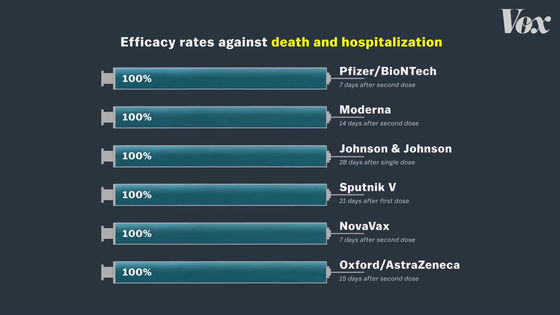
There are no serious cases or deaths in the vaccinated group.

The real purpose of the vaccine is to protect the human body from infections and to prevent serious symptoms such as hospitalization if they do. From that perspective, all COVID-19 vaccines are 100% effective.
Related Posts: