FDA approves Apple AirPods Pro 2 hearing aid function as 'world's first commercially available hearing aid software device'
It has been revealed that the Food and Drug Administration (FDA) has approved the hearing support feature designed for Apple's wireless earphones 'AirPods Pro' to be used as a hearing aid. This means that 'AirPods Pro 2' will be able to use the hearing aid function in a future software update.
FDA Authorizes First Over-the-Counter Hearing Aid Software | FDA
https://www.fda.gov/news-events/press-announcements/fda-authorizes-first-over-counter-hearing-aid-software
Apple AirPods Pro 2 have been approved by the FDA to function as hearing aids | TechRadar
https://www.techradar.com/audio/earbuds-airpods/apple-airpods-pro-2-have-been-approved-by-the-fda-to-function-as-hearing-aids
On September 9, 2024, Apple announced that it had designed a hearing aid feature called the 'Hearing Aid Feature' to help people with mild to moderate hearing loss, and that it would soon receive approval for use as a hearing aid. Three days after the announcement, the FDA released a release revealing that it had approved the feature as the 'first commercially available hearing aid software device.'
Apple's hearing aid function is the world's first 'software-based' function. It is intended to be used with AirPods Pro, and its unique feature is that it can add hearing aid functionality to existing products without modifying the device.
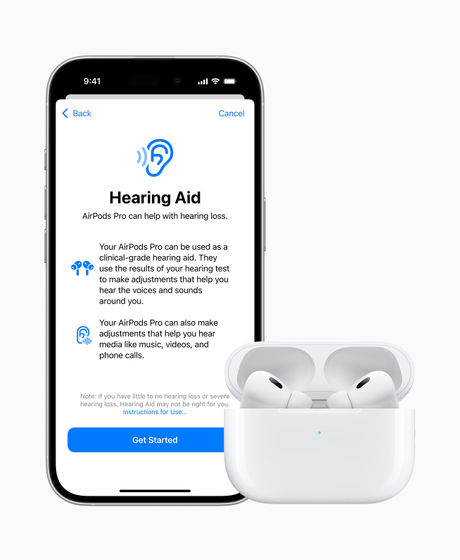
The hearing aid feature includes a clinically-based hearing test using iPhone and AirPods Pro, and then uses the information gained from the hearing test to turn AirPods Pro into a personalized hearing aid and noise protection. Once your AirPods Pro are personalized, you can take advantage of features like amplifying specific sounds around you.
The hearing aid feature will first be added to AirPods Pro 2. It will be available in more than 150 countries and regions, including Japan, the United States, and the EU, by September 2024. The hearing aid feature is intended for adult users aged 18 and over.

'Hearing loss is a significant public health issue affecting millions of Americans,' the FDA said. 'The approval of this hearing aid software add-on to a widely used consumer audio product marks a step toward making hearing assistance more available, accessible and acceptable to adults with mild-to-moderate hearing loss.'
Related Posts:







