Peer-reviewed paper on FDA-approved electronic tinnitus treatment device 'Lenire' published

A device called 'Lenire' that uses electrical stimulation on the tongue to treat tinnitus has been developed and approved by the U.S. Food and Drug Administration (FDA). The paper that led to the FDA's approval has been published after peer review.
Combining sound with tongue stimulation for the treatment of tinnitus: a multi-site single-arm controlled pivotal trial | Nature Communications
https://www.nature.com/articles/s41467-024-50473-z
Neuromod's Tinnitus Treatment Device Validated by Clinical Trial Results | The Hearing Review
https://hearingreview.com/hearing-products/tinnitus-devices/neuromods-tinnitus-treatment-device-validated-by-clinical-trial-results
It is estimated that tinnitus affects 10-15% of the population, with 2-8% of these suffering from severe symptoms. Despite the large number of sufferers, there are limited treatment options available, and so far treatments have included playing specific sounds.
One of the methods that has been proven to be effective in treating tinnitus is to stimulate the peripheral nerves. Several animal experiments and human clinical trials have confirmed that combining sound therapy with electrical stimulation of peripheral nerves such as the trigeminal nerve can improve the symptoms of tinnitus. Of these, it has been proven that 'electrical stimulation of the tongue' is the most effective at acting on the auditory cortex, which is thought to be the cause of tinnitus.
Lenire, developed by Neuromod Devices, is a device that treats tinnitus by applying electrical stimulation to the tongue in addition to sound.
Lenire uses a device with stainless steel electrodes that are placed in the mouth and pressed against the tongue.
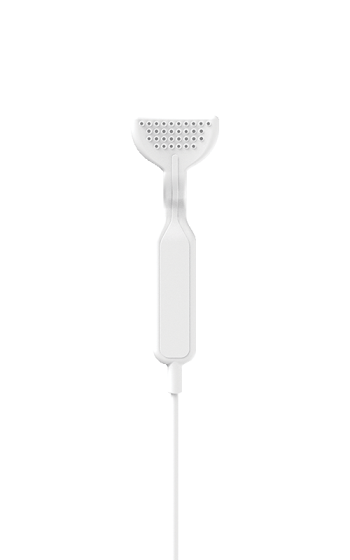
The device is connected to a controller that allows you to adjust the intensity of the stimulation.

Another thing is that there are headphones that are specially paired with the controller.

We try to treat tinnitus by stimulating the tongue and playing sounds.

Neuromod Devices has conducted two trials to obtain FDA approval, and using the findings from those trials, it has conducted a third, final large-scale clinical trial under the guidance of the FDA.
In the third test, for the first time, a comparison was made between 'sound alone' and 'sound and electrical stimulation,' and the results were compared with those of the first two tests.
The trial recruited 112 participants who were deemed to be sufficiently bothered by tinnitus. In the first phase, they received sound-only treatment for six weeks, and in the second phase, they received bimodal treatment, which combined sound-only and tongue stimulation, for six weeks.
The intensity of the electrical stimulation on the tongue was calibrated for each participant and set to the minimum electrical stimulation they could perceive on a scale from 'hardly perceptible' to 'clearly perceptible.' This intensity was saved as a calibrated setting, and participants were able to adjust the electrical stimulation on their tongue up or down six increments above and below the default level.
After each participant completed the course of treatment, they were surveyed in the final week to determine their satisfaction with the treatment and whether they would recommend Lenire treatment to other tinnitus sufferers.

The study found that bimodal sound and electrical stimulation treatment produced significant improvements compared to sound stimulation alone. Additionally, 62.9% of participants reported benefiting from using the Lenire device, and 88.6% would recommend the treatment to other tinnitus sufferers. No serious adverse events were reported.
The paper states, 'No participants discontinued the study due to device-related adverse events, and no device defects were reported. Interestingly, a higher proportion of patients would recommend the treatment than those who reported feeling a benefit, indicating high acceptability of the Lenire device and that participants were willing to recommend the treatment to others even without any guarantee of success. This high level of satisfaction and acceptability is consistent with the rate at which patients correctly adhered to the treatment protocol in this study.'
After this third round of testing, Lenire was successfully approved by the FDA and has already obtained the CE mark, which meets the standards of EU member states, making it available as a treatment option in some clinics in Europe as well as the United States.
Related Posts:
in Science, Posted by log1p_kr