A clinical trial of an mRNA universal influenza vaccine has started, and if it is put into practical use, it may be unnecessary to vaccinate every year
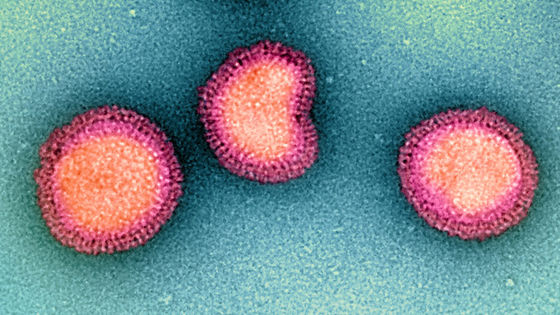
Subjects in a phase 1 clinical trial of the influenza virus mRNA vaccine 'H1ssF-3928 mRNA-LNP' developed by researchers at the National Institute of Allergy and Infectious Diseases (NIAID), an institution of the National Institutes of Health (NIH). Registration has started.
Clinical trial of mRNA universal influenza vaccine candidate begins | National Institutes of Health (NIH)

Seasonal influenza is prevalent not only in Japan but also in the United States every year, and according to the American Centers for Disease Control and Prevention, between 2010 and 2020, between 12,000 and 52,000 people died of influenza in the United States each year. It is estimated that
There are many types of seasonal influenza viruses, and which type is prevalent changes each time. So, before each flu season, we predict which types will be prevalent and select the influenza virus strains to incorporate into the vaccine. Pharmaceutical companies then manufacture and distribute the vaccine, during which time the dominant strain of the virus can change in unexpected ways, making the vaccine less effective.
The H1ssF-3928 mRNA-LNP developed by the NIAID research team is an influenza virus mRNA vaccine. Influenza virus envelopes contain a protein called hemagglutinin (HA). Although this HA mutates with the evolution of influenza viruses, there are almost no major mutations, and the HA of various types of influenza viruses is very similar. H1ssF-3928 mRNA-LNP is a system that induces immunity against this HA.
As such, H1ssF-3928 mRNA-LNP could be a 'universal influenza virus vaccine' that does not require strain prediction like traditional influenza virus vaccines, providing longer-lasting immunity and higher efficacy than traditional influenza virus vaccines, NIH said. I'm here.
by Government of Prince Edward Island
Enrollment for this clinical trial is recruiting up to 50 healthy subjects between the ages of 18 and 49. Subjects are divided into three groups and receive 10 micrograms, 25 micrograms, and 50 micrograms of vaccine. The study will also include a group receiving the current quadrivalent seasonal influenza vaccine. In addition, in order to evaluate the safety of the vaccine, it is planned to regularly screen subjects for up to one year after vaccination.
'A universal influenza virus vaccine would be a major public health achievement that would eliminate the need to develop a seasonal influenza virus vaccine each year, and patients would not need to be vaccinated against influenza every time,' said NIAID Acting Director Hugh Auchinkloss. 'Also, some types of influenza can cause pandemics, and a universal influenza vaccine could serve as an important defense against future spread of influenza pandemics.' .
Related Posts:
in Science, Posted by log1i_yk