Looking for Healthy Volunteers for Clinical Trial of World's First New Coronavirus Vaccine
Pharmaceutical companies are developing vaccines to combat the
Safety and Immunogenicity Study of 2019-nCoV Vaccine (mRNA-1273) to Prevent SARS-CoV-2 Infection-Full Text View-ClinicalTrials.gov
https://clinicaltrials.gov/ct2/show/NCT04283461
mRNA Coronavirus Vaccine Study: KPWHRI
https://corona.kpwashingtonresearch.org/
First coronavirus vaccine trial in the US is recruiting volunteers | Live Science
https://www.livescience.com/us-coronavirus-vaccine-trial-recruiting.html
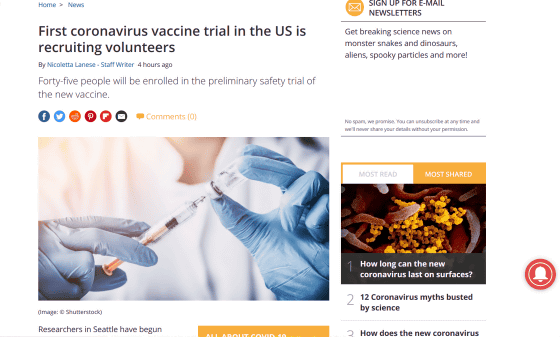
Forty-five subjects will be divided into three groups, each with a different dose of vaccine, and will receive a total of three doses of vaccine at 28-day intervals. Following the administration, the follow-up will be continued for almost a year, and the volunteers will need to make a total of 11 face-to-face research visits during the study period. The volunteers will receive $ 100 (about 16,000 yen) for each visit, a total of $ 1100 (about 106,000 yen). According to ClinicalTrials.gov, the clinical trial is expected to end on June 1, 2021.
According to a report from
Instead of using viruses, new vaccines contain genetically-generated messenger RNA (mRNA) , a genetic material that plays a role in synthesizing proteins according to genetic information. The artificial mRNA contained in the vaccine seems to encourage the production of 'proteins on the surface of the new coronavirus' in the recipient's body.
Eventually, the vaccine mRNA is degraded and removed from the recipient's body. On the other hand, it has been described that the human immune system produces antibodies in response to this newly produced protein, so that vaccination can provide immunity against the new coronavirus.
While vaccine development is advancing, there is no guarantee that a new coronavirus vaccine will be low enough for everyone to buy.
Meanwhile, Stéphane Bancel, CEO of Moderna Therapeutics, believes that the developed vaccines should be offered at low prices. 'We are very aware that this is a public health problem, so if a vaccine is approved, pricing will be deeply considered. Price higher than vaccines for other respiratory tract infections It should not, 'said Bansel.

Related Posts:
in Science, Posted by log1h_ik