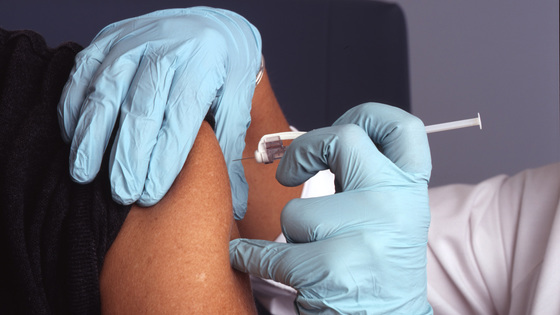
World's first human clinical trial of new coronavirus vaccine started
On March 16, 2020 local time, the National Institutes of Health (NIH) announced that it has started human clinical trials of a new coronavirus vaccine. The new coronavirus vaccine, which has entered the human clinical trial stage, is the world's first.
NIH clinical trial of investigational vaccine for COVID-19 begins | National Institutes of Health (NIH)
https://www.nih.gov/news-events/news-releases/nih-clinical-trial-investigational-vaccine-covid-19-begins
US Just Started The First Human Trial of a Vaccine For The New Coronavirus
https://www.sciencealert.com/us-begins-first-human-trial-of-coronavirus-vaccine
Entering the human clinical trial phase is a vaccine called mRNA-1273, co-developed by NIH and Moderna Therapeutics , a biotechnology company based in Cambridge, Mass. When this vaccine is administered, the protein on the surface of the new coronavirus is produced in the recipient, and antibodies against the new coronavirus are produced against the protein. As a result, the recipients are less likely to be infected with the new coronavirus and can be prevented from becoming more severe.
NIH was recruiting subjects for 'mRNA-1273' in the United States as of March 6, 2020.
Looking for healthy volunteers for clinical trial of world's first new coronavirus vaccine-gigazine
On March 16, 2020, NIH announced that it would begin a six-week open -label study to secure 45 healthy volunteers between the ages of 18 and 55. This clinical trial, called a phase I trial , is intended to verify the efficacy and safety of a small population. The NIH states that intramuscular injections of the upper arm administer different volumes to each group of subjects to confirm safety and the degree to which they elicit an immune response. Will be checked.
Because no vaccine or treatment for the new coronavirus infection (COVID-19) approved by a public agency exists at the stage of writing, the development of “mRNA-1273” is promising. However, it is reported that it will take one year to one and a half years to commercialize `` mRNA-1273 '' because multiple human clinical trials still have to be performed to confirm the efficacy and safety .
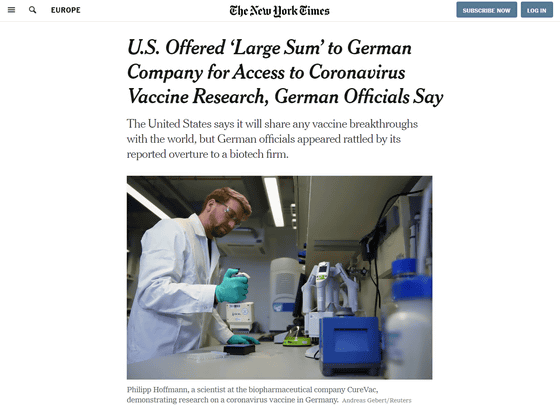
Research and development of new coronavirus vaccines are overheating in various countries in response to the spread of the new coronavirus epidemic. One report states that 'President Trump of the United States has funded $ 1 billion to German biopharmaceutical company CureVac , which is currently developing a vaccine for a new coronavirus.' However, this funding has raised a backlash from Germany and the EU, saying that there is a requirement to 'ship vaccines to the United States.'
US Offered 'Large Sum' to German Company for Access to Coronavirus Vaccine Research, German Officials Say-The New York Times
https://www.nytimes.com/2020/03/15/world/europe/cornonavirus-vaccine-us-germany.html
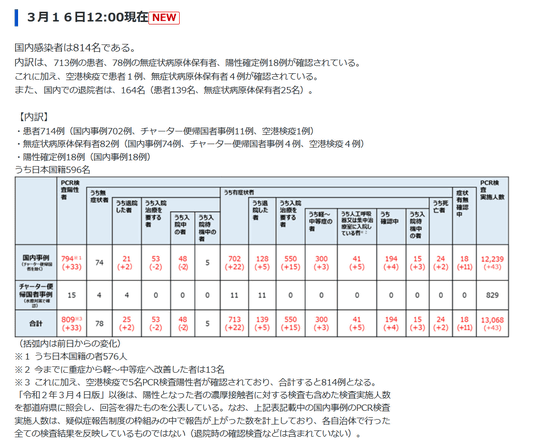
COVID-19 is rapidly spreading in countries such as the EU, far from its source, Wuhan. The Ministry of Health, Labor and Welfare has announced that the number of infected people in Japan as of March 16, 2020 is '814 domestically infected people'.
About New Coronavirus Infections
https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000164708_00001.html
Related Posts:
in Note, Posted by darkhorse_log