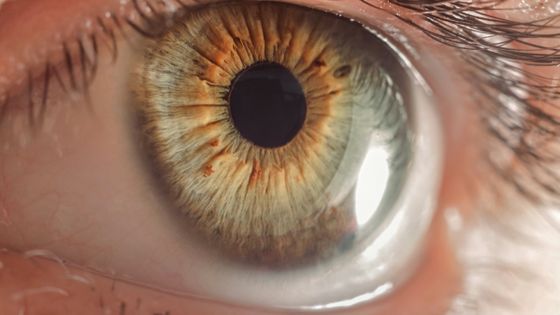
Blind patients using electronic retinal implants are at risk of losing artificial vision as the technology becomes outdated and unsupported
Advances in technology have led to the emergence of technology that restores the eyesight of blind people. One of them, the company that provided the electronic retinal implant technology, was absorbed and merged with another company due to the management crisis, and the technology up to that point was also abandoned, so the electronic retinal implant is already embedded and used. IEEE Spectrum reports that people's support has virtually ended.
Their Bionic Eyes Are Now Obsolete and Unsupported --IEEE Spectrum
Go read this dystopian story about patients whose bionic eyes went obsolete --The Verge
https://www.theverge.com/2022/2/16/22937198/bionic-eye-company-defunct-ieee-spectrum-go-read-this
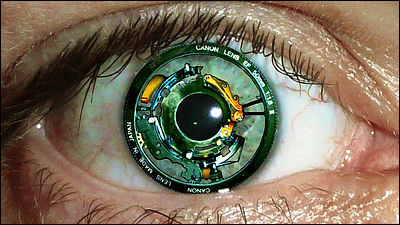
At issue is Second Sight Medical Products , a company that develops electronic retinal implant technology. Second Sight Medical Products is a company launched in 1998 by Robert Greenberg, who turned from an electrician to a doctor. Based on the results, we have developed the electronic retinal implant 'Argus II'.
US Food and Drug Administration approves 'Argus II', an artificial retinal device that supports the vision of people with severe blindness or severe visual acuity --GIGAZINE
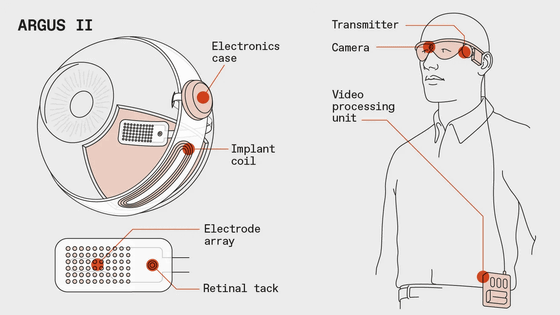
Argus II consists of glasses with a camera and a video processing unit (VPU) attached to the belt. The image captured by the camera attached to the glasses is processed by the VPU, converted into a 60-pixel monochrome pattern, then sent to the transmitter attached to the glasses and sent to the 60 electrodes attached to the retina to stimulate the retina. A mechanism to make it visible.
The price of Argus II is 150,000 dollars (about 1.7 million yen) per unit, which is about 5 times the price of other neural devices. Including surgery and rehabilitation, it costs $ 497,000 (about 57 million yen) for one eye alone.
The images that can be seen on the Argus II are not colorful and high resolution, and it is difficult to even grasp the shape and appearance of an object because it is a black and white image of only 60 pixels, but Ross lost his eyesight in one eye and underwent surgery. 'It's not normal vision, but it's still 100% better than your current vision,' said Dore.

However, Second Sight Medical Products stopped manufacturing electronic retinal implants in 2019 and was in danger of going bankrupt in 2020. After that, it announced that it would conduct a clinical trial of the intracerebral implant 'Orion' to obtain artificial vision, and raised $ 57.5 million (about 6.64 billion yen) through a public offering, but it was $ 5 (about 580 million yen). The stock price, which was (yen), fell to $ 1.5 (about 170 yen), and Second Sight Medical Products was merged with the biopharmacy company Nano Precision Medical.
According to IEEE Spectrum, there are more than 350 people worldwide who have had Argus II surgery, and Second Sight Medical Products has improvements and software to increase the video resolution of Argus II for those who have had surgery. He promised technical future such as updating of. However, with Greenberg resigning as CEO during the 2020 bankruptcy crisis and most employees being fired, it's almost impossible to update Argus II.

Barbara Campbell, who implanted the implant in an Argus II clinical trial, has been using it for four years since surgery, but in 2013 the system shut down suddenly while walking in a subway station. Even if I sent the main unit for repair, there was no sign that it would be cured, and Campbell's Argus II never worked again. After that, he consulted with his doctor about surgery to remove the implant from the retina, but he concluded that it was not worth removing the implant considering the risks. As a result, the electrodes that have lost their function remain in Campbell's left eye.
Other cases similar to Campbell have been reported, and a post-approval study by the US Food and Drug Administration (FDA) found 36 serious and 152 adverse events observed in the IEEE Spectrum. Is reporting. In addition, some people who underwent Argus II implant surgery had retinal detachment.
In addition, Second Sight Medical Products is developing a vision recovery technology using a brain implant called 'Orion' in 2020, but Nano Precision Medical, which merged with Second Sight Medical Products, said, 'We have any plans for Orion. We haven't done that, 'and we expect that Orion will no longer be supported as a result.
IEEE Spectrum says, 'Innovation is a failure. Argus II is an innovative technology, and Second Sight Medical Products may have been ahead of other companies developing artificial vision systems, but in the future. For those considering electronic retinal implants, the lesson that Argus II patients have been neglected may slow down that decision. Should we challenge innovative techniques? Is it okay to allow us to depend on it, even if it shows us the world? '
Related Posts: