A remedy for smallpox that should have been eradicated is approved, due to concerns that it will be used as a biological weapon
On June 4, 2021, the US Food and Drug Administration (FDA), which regulates medical products in the United States, approved the treatment for smallpox 'Tembexa (brinside fobyl )'. The smallpox virus was declared eradicated in 1980, and it is said that it no longer exists in nature, but there are concerns that it may be used as a chemical weapon in the future.
FDA approves drug to treat smallpox | FDA
https://www.fda.gov/drugs/drug-safety-and-availability/fda-approves-drug-treat-smallpox
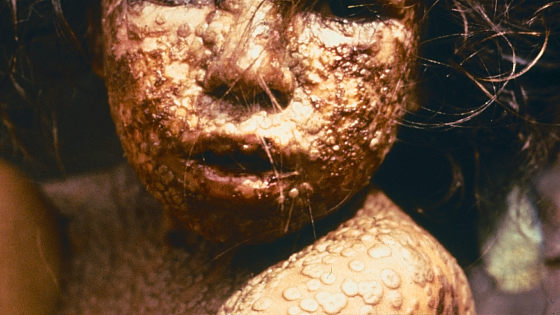
Smallpox is an infectious disease that has strong infectivity to humans and causes symptoms such as high fever, headache, and low back pain in infected people. The name comes from the fact that infected people develop bean-grained papules on their heads and faces that are slightly whiter than the skin and spread throughout the body. The case fatality rate is as high as 20 to 50% on average, and it has been prevalent all over the world in the past, causing many deaths.
Symptoms of smallpox can be seen in the following articles.
If you reproduce the symptoms of a disease that has caused many deaths in history with special make-up, it will be like this --GIGAZINE

On May 8, 1980, WHO issued the 'Declaration of Eradication of Smallpox from Earth', which declared that the smallpox virus no longer exists in nature. On the other hand, smallpox virus is stored in facilities in the United States and Russia for research purposes.
Even if the virus does not exist in nature, there is always a risk of the virus leaking from the above research facilities. Although the source of the new coronavirus (COVID-19), which is prevalent worldwide, is not yet known, it is thought that it may have leaked from the Wuhan Institute of Veterinary Medicine in China.
What is the battle until the new coronavirus 'Wuhan Institute of Vessel Emanationism' moves the world? --GIGAZINE

In addition, smallpox has been designated by the US government as an ' infectious disease with a high possibility of bioterrorism.'
Against this background, the development of medical products for smallpox virus was judged to be important, and the FDA approved the smallpox treatment 'Tembexa (brinside phobil)' on June 4, 2021.
Approved by, Tenbekisa performs the high development of new drugs of the need to preferentially ' Fast track is applied', orphan drug will be subjected to the specified. For regular drugs, the application will be reviewed in 10 months or more, but this measure will allow Tenbexa to be reviewed in 6 months. Also, given the seriousness of the illness, the experiment was allowed to proceed promptly.
Since smallpox does not exist in nature, the effectiveness of the development of Tenbexa was confirmed by a virus closely related to smallpox virus. Experiments with animals have shown that animals treated with Tempexa have a higher survival rate than animals treated with placebo. On the other hand, because the animal efficacy rules have been applied to Tenbexa, the results of the study's effectiveness are based on the premise that 'ethics and feasibility are not sufficient for conducting clinical trials in humans.' Will be accepted.
Related Posts:
in Science, Posted by darkhorse_log