Clinical trials may underestimate serious side effects because high-risk patients are less likely to participate.

The safety of new drugs is judged based on the results of clinical trials. However, if the patients participating in clinical trials do not match the 'patient profile of the patients who will actually use the drug,' the risk of serious side effects may appear small. A working paper by the National Bureau of Economic Research (NBER) used the example of cancer treatment drugs to show that 'patients more likely to experience serious side effects tend to be less likely to participate in clinical trials,' and reported that estimating the harm of a drug based solely on clinical trial data may result in an underestimated figure.
Trials Avoid High Risk Patients and Underestimate Drug Harms | NBER
A research team led by Jason Avallack of Yale University, Leila Agha of Harvard Medical School, and Sachin Shah of Massachusetts General Hospital focused on the question of 'what kind of patients are the data collected from in clinical trials?' Clinical trials impose participation requirements, so people with many complications or chronic illnesses or who are physically weak are often excluded, or find it difficult to participate due to the burden of paperwork and medical visits. The research team pointed out that this means that clinical trial participants tend to be relatively healthy patients.
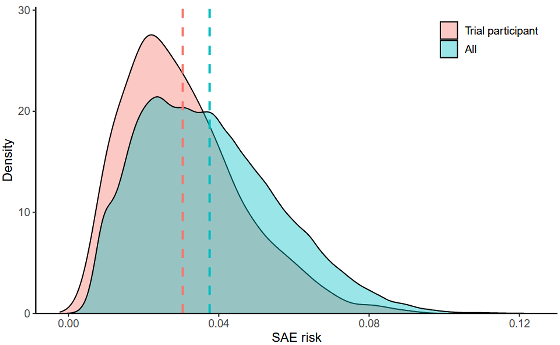
The research team considered that this bias could affect the perception of safety, and looked at the increase in 'hospitalization
The research team thought, 'If hospitalizations increase immediately after treatment begins, it's possible that events suspected to be related to the drug are occurring around the same time.' They matched cancer registry data with medical insurance claims data to track when patients began taking the drug and their hospitalization records, and then compared the increase in hospitalizations before and after treatment began.
The researchers found that there was a trend toward increased hospitalizations for SAEs after starting cancer treatment, with an average risk increase of 2 percentage points per month, equivalent to two more hospitalizations per 100 patients per month.
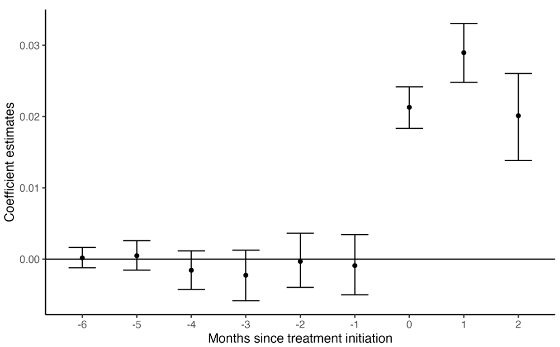
The research team also confirmed whether this increase occurred at the same rate for everyone. The researchers explained that the likelihood of hospitalization due to SAEs can be predicted to some extent by characteristics such as the number of comorbidities and
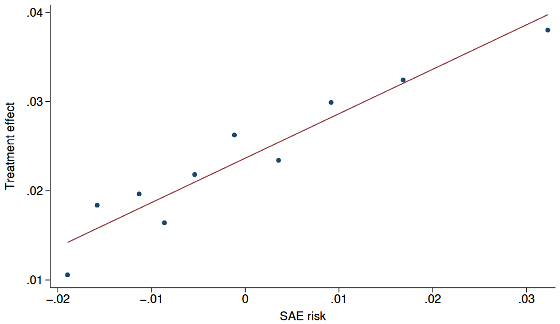
However, the researchers also found that higher-risk patients tended not to participate in clinical trials. The researchers reported that patients in the top 10% of the risk distribution were estimated to be about 2.5 times more likely to be hospitalized due to an SAE after starting treatment than those in the bottom 10%, but were about one-quarter as likely to participate in clinical trials.
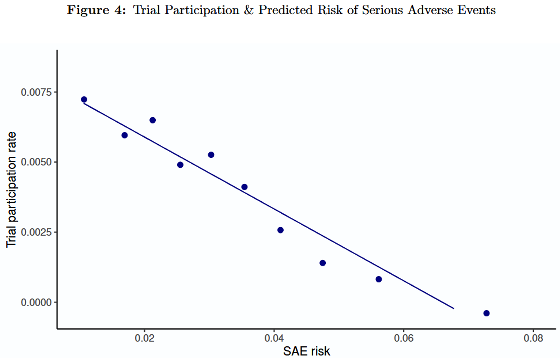
The research team also estimated how this bias might affect the perception of safety. As a result, the expected increase in hospitalizations due to drug-related SAEs in the target population for whom the drug is actually prescribed could be 15% larger than the estimate based on clinical trial participants alone. The estimate suggests that after one year of treatment, 'there would be approximately one additional hospitalization due to an SAE thought to be due to the drug for every 25 patients.'
The research team pointed out that the U.S. Food and Drug Administration (FDA) has not formally defined detailed rules such as 'ensuring that clinical trial participants closely resemble real-life patients.' They also stated that if patients who are more likely to experience serious side effects drop out of a clinical trial, the number of SAEs seen in the trial may be smaller, leading to an optimistic estimate of the drug's harm.
This research has also been discussed on the social news site Hacker News, with comments such as, ' In trials with a small number of cases, differences in conditions can fluctuate the results, so it is more realistic to expand the subjects in more advanced trials, ' ' Clinical trial procedures are very strict, and even the slightest deviation can invalidate the results ,' ' There is an idea to track patients after approval, but it is difficult because there is little motivation to collect data ,' ' There is a system in place for reporting side effects, and reports are collected in a database ,' and ' A relative with a terminally ill cancer tried to find a place to participate in a clinical trial but was unable to find one .'
Related Posts:
in Science, Posted by log1b_ok