A new device has been developed to extract 'green ammonia' from the air, which can be used as fuel or fertilizer

The Haber-Bosch process, which made it possible to mass-produce chemical fertilizers, supports agriculture around the world, but it is also an energy-intensive technology that accounts for more than 2% of global energy consumption and about 5% of natural gas consumption, so there are sustainability issues. A paper published in the scientific journal Science Advances on December 13, 2024 announced a device that collects ammonia from the air, which can be used as plant fertilizer.
Onsite ammonia synthesis from water vapor and nitrogen in the air | Science Advances
Stanford Researchers Produce Ammonia Fuel From Thin Air - IEEE Spectrum
https://spectrum.ieee.org/ammonia-fuel-2670794408
Ammonia is the main component of agricultural fertilizers, but in recent years it has also been considered important as a sustainable fuel: it can be decomposed to produce hydrogen for fuel cells and internal combustion engines, and when hydrogen is burned it produces only water without emitting carbon.
Ammonia is easier to handle than gaseous hydrogen because it dissolves easily into liquid form, making it less expensive per kilowatt-hour. The shipping industry is already turning to ammonia, with the world's first zero-carbon ammonia-powered ship due to launch in 2024.
Focusing on ammonia as a cleaner fuel than methane or hydrogen gas, a team from Stanford University and Saudi Arabia's King Fahd University of Petroleum and Minerals has developed a green ammonia collection device that uses nitrogen and water vapor from the air.
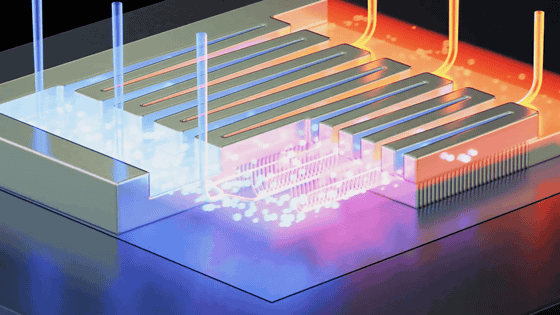
Below is a schematic diagram of the 'on-site ammonia production unit' presented by the research team. The unit consists of a catalytic mesh, a cooling condenser plate for collecting the collected liquid sample, and a collection vessel.
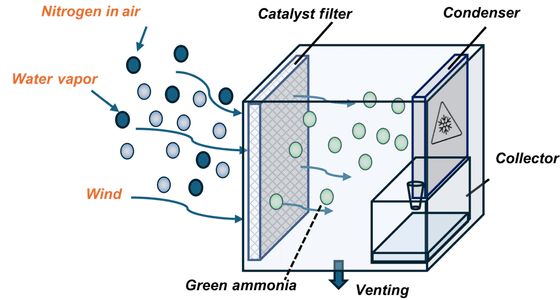
When water vapor and nitrogen in the air are passed through a catalytic mesh, either by natural wind or a fan built into the device, green ammonia is generated inside the device, and this is then cooled, condensed, and collected as a liquid.This is how the on-site ammonia production device works.
Ammonia is produced by a process in which water vapor (H2O) and atmospheric nitrogen (N2) are combined with a catalyst made of magnetite and a polymer called
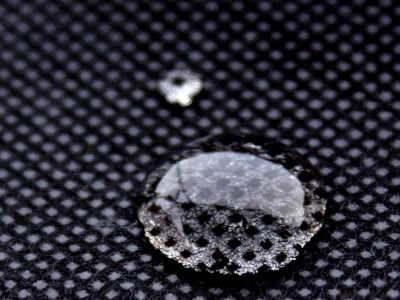
Below is a photo of the device on the Stanford University campus. Portable power is used for the cooling process to collect ammonia.

In their experiments, the team was able to achieve ammonia concentrations of up to 120 μM (micromolar) per hour, which is enough for some crops, and can also achieve higher concentrations if needed by using zeolites, a material commonly used to remove ammonia from wastewater.
'This innovation has the potential to fundamentally change fertilizer production by providing a sustainable, cost-effective alternative to intensive production,' said Richard Zare, a professor of chemistry at Stanford University and one of the paper's authors. 'It also has the potential to revolutionize chemistry in everything from intensifying agriculture in developing countries to pharmaceutical manufacturing and industrial advances.'
Related Posts:
in Science, Posted by log1l_ks