A new nitrogen fixation method to create a pharmaceutical material from nitrogen in the air and crude oil is developed

A research team at Yale University announced that it has developed a new method of
Coupling dinitrogen and hydrocarbons through aryl migration | Nature
https://www.nature.com/articles/s41586-020-2565-5
New nitrogen products are in the air | YaleNews
https://news.yale.edu/2020/08/12/new-nitrogen-products-are-air

Nitrogen fixation and the nitrogen molecules present in the air (N 2), ammonia (NH 3) and nitrate (NO 3 -) the process of converting the high nitrogen compound reactive like. In the natural world, microbes in the ground absorb nitrogen in the air and convert it into forms such as glutamate and nitrate that can be used by plants.
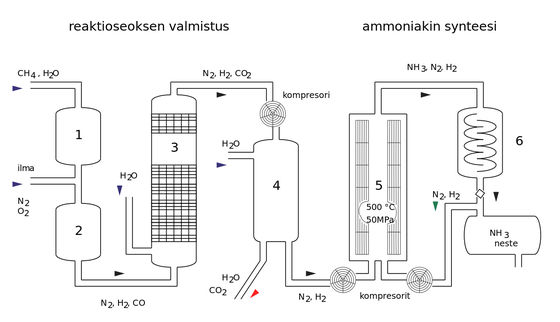
The Haber-Bosch method, which was developed in the early 20th century, is a method of artificially fixing nitrogen. The Haber-Bosch method, which uses an iron catalyst and a high-temperature and high-pressure device to break the triple bonds of nitrogen molecules to synthesize ammonia as a chemical fertilizer, was called 'a method of making bread from water, coal, and air.'
by Antimoni
A research team led by Professor Patrick Orland of the Department of Chemistry at Yale University announced that nitrogen in the atmosphere and benzene contained in crude oil will be combined with iron compounds and silicon compounds to become materials for dyes and pharmaceuticals. This is a nitrogen fixation method in which the derivative of aniline is purified.
In the conventional industrial synthesis of aniline, the mainstream method is to react benzene with concentrated sulfuric acid and concentrated nitric acid to first synthesize highly toxic nitrobenzene and then reduce it to aniline. However, if aniline could be made from nitrogen in the air and benzene, it would be unnecessary to synthesize toxic nitrobenzene.
There have been attempts to synthesize aniline by reacting nitrogen with benzene. However, according to Professor Orland, a very reactive benzene derivative was used in the previous research, and it failed because it decomposes before it reacts with nitrogen.
'In the long run, I would like to study how to use nitrogen, which is abundant in the atmosphere, as a resource for synthesizing products that society needs,' commented Holland.
Related Posts:
in Science, Posted by log1i_yk







