A process to convert carbon dioxide into fuel with high efficiency of over 90% has appeared, and conversion to formate makes it a solid fuel that can be stored for a long time.

Various studies are being conducted to convert carbon dioxide into useful substances, and one of the most promising is converting it into a stable fuel that can be used in place of fossil fuels. However, this type of conversion process has a number of problems, including low efficiency, difficult handling, toxicity, and flammable fuels.
Researchers at the Massachusetts Institute of Technology and Harvard University have developed a process that overcomes these problems and efficiently converts
A carbon-efficient bicarbonate electrolyzer: Cell Reports Physical Science
https://www.cell.com/cell-reports-physical-science/fulltext/S2666-3864(23)00485-X
Engineers develop an efficient process to make fuel from carbon dioxide | MIT News | Massachusetts Institute of Technology
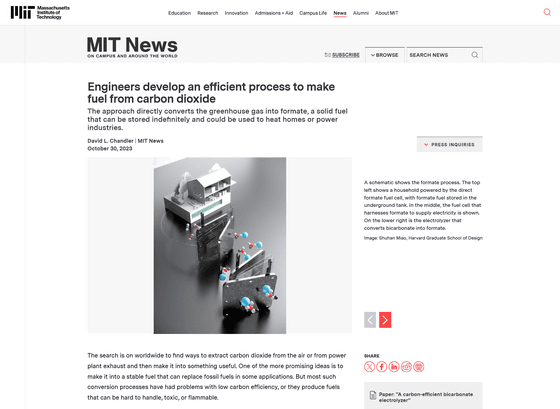
According to MIT professor Ju Li, who is involved in the research, the process of converting carbon dioxide into fuel is to first synthesize carbon dioxide with calcium and solidify it into calcium carbonate, then heat it and emit the carbon dioxide. Two steps are required: converting it into carbon monoxide, which is the raw material for fuel. The conversion efficiency of the second stage is particularly low, with less than 20% of the gaseous carbon dioxide being converted into the desired fuel raw material.
The key to the new process is that the first step converts carbon dioxide to an intermediate form, liquid metal bicarbonate, eliminating the need for inefficient heating. Liquid metal bicarbonates can be electrochemically converted into potassium or sodium formate solutions in electrolyzers using low-carbon electricity such as nuclear, wind, or solar power. By drying this liquid, a stable solid powder can be obtained. The conversion efficiency is said to be as high as 90%.
This time, the research team electrochemically converted liquid metal bicarbonate using a cation exchange membrane electrolyzer. We confirmed that it can be converted to solid crystals of formate with an efficiency of over 96%.
One candidate for converting carbon dioxide into fuel is methanol, which consumes carbon dioxide during production, but methanol is a hazardous substance and can pose health risks in the event of a leak. On the other hand, formate has been determined to be safe under American safety standards.
Up until now, this type of system has faced a number of challenges. This time, the research team has refined the design of the membrane's materials and composition to solve the problem that this type of system inevitably suffers from: the buildup of certain byproducts changes the pH and reduces efficiency over time. Cleared by keeping pH in a steady state using mechanical modeling. In tests, there was no significant decrease in output even after the system was operated for 200 hours.
Additionally, the problem of ``undesirable side reactions producing other, useless chemical products'' can be addressed by adding a buffer layer of bicarbonate-rich fiberglass wool that blocks these reactions. We have succeeded in preventing side reactions.
The research team is also building a fuel cell optimized to use the produced formate fuel to generate electricity. The mechanism is that formate particles are dissolved in water and pumped into the fuel cell as needed. Considering the weight and volume of the high-pressure tanks required to store hydrogen, Lee explains that formate fuels produced roughly the same electrical output for a given storage volume.
Related Posts:
in Science, Posted by logc_nt