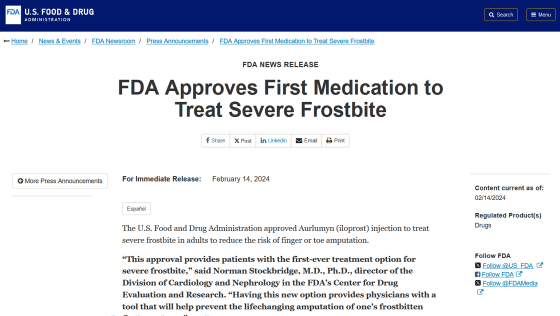
U.S. Food and Drug Administration approves first drug to treat severe frostbite that can cause finger and toe amputation

FDA Approves First Medication to Treat Severe Frostbite | FDA
https://www.fda.gov/news-events/press-announcements/fda-approves-first-medication-treat-severe-frostbite
First frostbite treatment approved by FDA | CNN
https://edition.cnn.com/2024/02/14/health/iloprost-frostbite-fda/index.html
1st frostbite drug approved by FDA after successful clinical trial | Live Science
https://www.livescience.com/health/medicine-drugs/1st-frostbite-drug-approved-by-fda-after-successful-clinical-trial
Frostbite, which mainly involves freezing of tissues such as limbs, ears, and nose, can occur during winter mountain climbing. In mild cases, the condition only causes loss of skin sensation, discoloration of the affected area, and a stinging sensation, and can be treated by warming the affected area at room temperature or with warm water. However, when the disease becomes so severe that the skin becomes hard and frozen, the affected area becomes necrotic due to low oxygen conditions due to poor blood circulation, and there are cases where the rotting affected area has to be amputated.
Cases of severe frostbite are rare, and it is estimated that only 1 in 100,000 people in the United States suffered severe frostbite from 2016 to 2018. However, the consequences of amputation can have lifelong consequences.
On February 14, 2024, the FDA approved a new vasodilator drug called iloprost for the treatment of severe frostbite. 'This approval provides patients with the first-ever treatment option for severe frostbite,' said Dr. Norman Stockbridge of the FDA's Center for Drug Evaluation and Research. 'We provide doctors with the tools to prevent this from changing.'

Iloprost is a drug that widens blood vessels to improve blood flow and prevents blood from coagulating within the blood vessels, and is already used to treat
In an open-label clinical trial of 47 adults with severe frostbite, ``a group receiving iloprost alone'' and ``a group receiving iloprost in combination with another drug'' in addition to the standard treatment acetylsalicylic acid (aspirin) were found. They were divided into three groups: a group that received only another drug, and a bone scan to predict the need for finger amputation seven days after frostbite.
0 out of 16 people (0%) in the group who received iloprost alone were judged to have a high risk of finger amputation based on bone scan findings, and 0 out of 16 people in the group who received iloprost in combination with another drug. The incidence was significantly lower in the group receiving iloprost, with 3 (19%) and 9 out of 15 (60%) in the group receiving only drugs other than iloprost. A follow-up study also found that the bone scan findings closely matched whether or not the affected area was actually amputated.

There are not many effective treatments for severe frostbite, and other clot-busting drugs carry a high risk of bleeding or are only effective within 24 hours of frostbite. However, iloprost does not have this risk of bleeding and can be used within 3 days after frostbite occurs, and is already being used as a treatment for severe frostbite in countries such as Canada, Europe, and Nepal.
'In my opinion, this is a game changer. This is a huge step forward in the treatment of frostbite in America,' said Dr. Peter Hackett of the University of Colorado Anschutz Medical Campus. However, Mr. Hackett argued that frostbite is an injury that should be prevented in the first place, so when doing outdoor activities in the cold season, you should prepare appropriate equipment and clothes and train in advance.
According to the FDA, side effects of iloprost include headache, palpitations, nausea, dizziness, and low blood pressure.
◆Forum now open
A forum related to this article has been set up on the GIGAZINE official Discord server . Anyone can write freely, so please feel free to comment! If you do not have a Discord account, please create one by referring to the article explaining how to create an account!
• Discord | 'Have you ever had frostbite?' | GIGAZINE
https://discord.com/channels/1037961069903216680/1209788703765958727
Related Posts:
in Science, Posted by log1h_ik