What is the most efficient and ideal engine 'Carnot cycle'?

Modern society is constantly supported by the conversion of energy. For example, most power generation systems convert thermal or potential energy into rotational energy and then into electrical energy. Also, it can be said that the battery in the smartphone converts chemical energy into electrical energy. The study of energy and
The Most Misunderstood Concept in Physics-YouTube
In 1813, Napoleon's failed campaign against Russia led to a coup d'état in Paris, France. Prussia and Austria then invaded France. Among the troops guarding Paris at the time was Sadie Carnot .

Carnot's father was Napoleon's general
His father, Lazar Carnot, was a military man and a physicist. Carnot is said to have had a great deal of discussion with his father about the steam engine, which was cutting edge at the time. Due to political instability in France at the time, France was far behind Britain in terms of industrial technology. Carnot realized that the steam engine could only convert about 3% of the heat energy into work, and thought that if the work efficiency of the steam engine could be improved, France would be able to make up for the delay. .

And Carnot's theory of efficient thermodynamic engine is 'Carnot cycle'. The Carnot cycle assumes a piston filled with
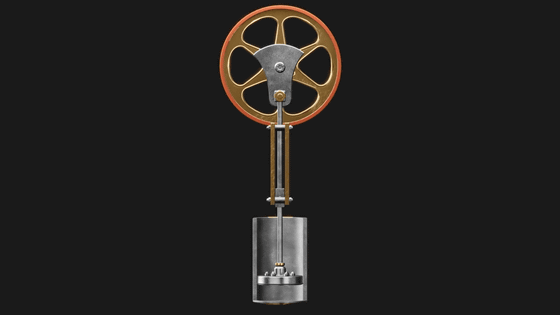
When the piston is placed on a hot metal plate, the gas inside the piston heats up and expands, pushing the piston up and spinning the flywheel.

Even if the heated metal plate is removed in the middle, the flywheel continues to rotate, so the piston continues to be pushed up and the gas continues to expand. However, since there is no heat inflow, the gas inside the piston expands and cools down.

Then, when the piston is placed on a cold metal plate, the gas inside the piston cools and contracts, causing the piston to move downward. At the same time the gas is compressed and heat escapes to the metal plate.
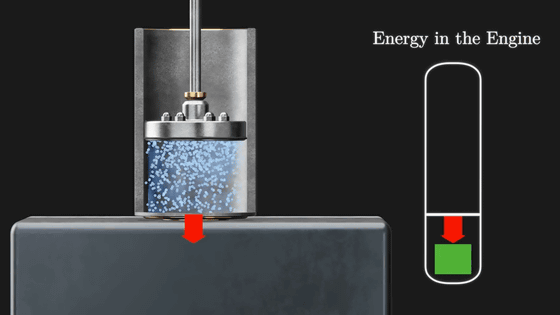
Then, when the cooled metal plate is removed, the force of the flywheel pushes the piston down further, compressing the gas, and this time the temperature of the gas inside the piston rises, returning to the initial state.
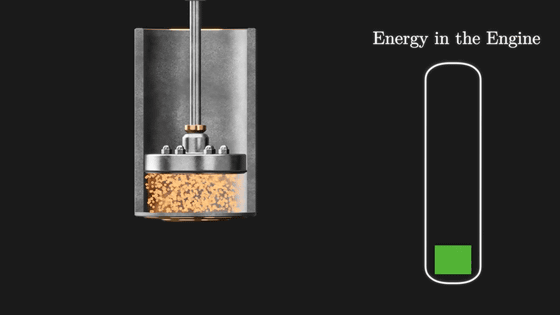
The Carnot cycle efficiently converts the heat applied from the outside into rotational energy of the flywheel by repeating these four stages. The point is that this Carnot cycle only efficiently changes the heat energy given from the outside into rotation, and finally returns to the initial state. However, its energy conversion efficiency is not 100%. The rotational energy (E) of the flywheel is the amount obtained by subtracting the 'heat amount flowing out to the cooled metal plate (Q C )' from the 'heat amount flowing in from the heated metal plate (Q H )'.
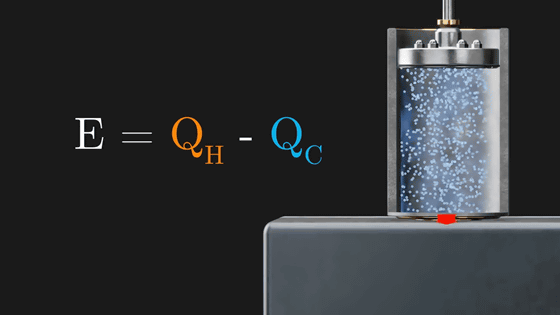
And the conversion efficiency is ``how much heat added to the Carnot cycle is converted to rotational energy'', so it can be obtained by E / (Q H ). As long as Q C exists, E will never be 1, i.e. 100%.
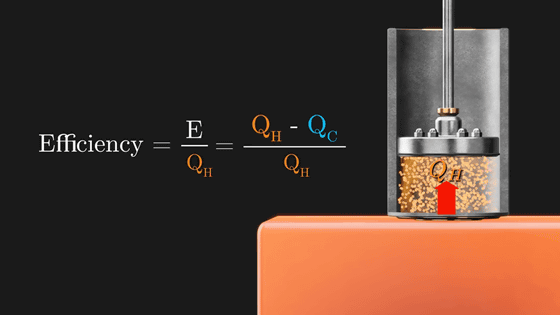
Q H corresponds to the work done by the gas to push the piston up (∫F H dx), and Q C corresponds to the work done by the gas to push the piston down (∫F C dx).
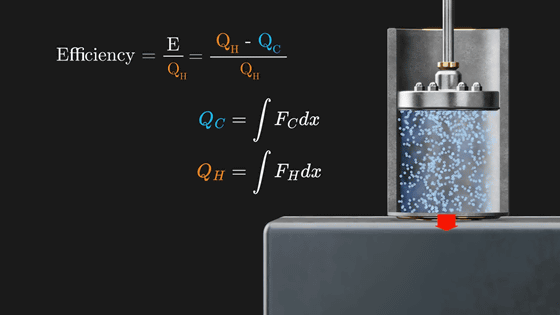
And when the gas is warmed by placing it on a hot metal plate, Q H will always be greater than Q C because the gas in the piston exerts more pressure on the piston than when it was cold. And in order to increase the efficiency of the engine (E/Q H ), it is necessary to increase the temperature difference between high and low temperatures.
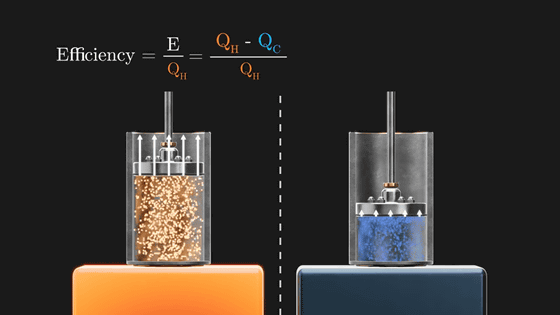
William

If the gas particles stopped moving, the work done by the gas to push the piston down, that is, QC , would be 0, so the efficiency of the Carnot cycle would be 100%. This 'state in which gas particles practically stop moving' is the concept of absolute zero.

However, the Carnot cycle is just an ideal and cannot be realized in reality. This is because heat must be thrown away to return the piston to its original position, and not all the energy is converted into rotation of the flywheel. The gas in the piston does not behave like an ideal gas, and the actual piston and flywheel have friction, and heat is diffused to the surroundings. Once the heat is transferred elsewhere, it cannot be recovered. In other words, in reality, it is impossible to return to the same state as the beginning after completing the four stages.
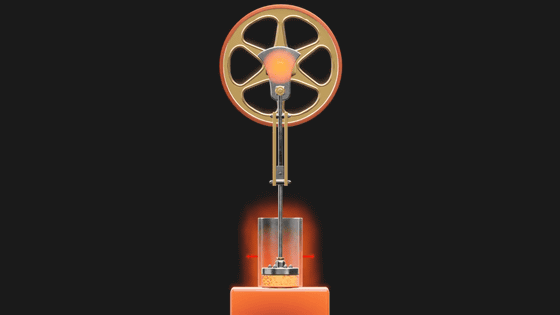
Energy is easiest to use when it is concentrated and least usable when it is diffused. German physicist

The reason why hot food cools down when left unattended and the fact that chilled tea gradually becomes lukewarm is because the energy of heat spreads over time. Clausius has published a series of research results in the form of '

Although the Carnot cycle itself is impossible to realize, the `` Stirling engine '' devised by Robert Stirling, a pastor in Scotland in 1816, is known to be quite close to this Carnot cycle. You can see what the Stirling engine is like by watching the following movie.
Stirling Engine-How It Works In Simple Terms-YouTube
Related Posts: