Research begins to confirm the effectiveness of combining different types of new corona vaccines
Vaccines for the new coronavirus are being developed by research institutes around the world. At the time of writing the article
World-first COVID-19 alternating dose vaccine study launches in UK --GOV.UK
https://www.gov.uk/government/news/world-first-covid-19-alternating-dose-vaccine-study-launches-in-uk
UK government to test mixing COVID vaccines in new trial --ABC News
https://abcnews.go.com/Health/uk-government-test-mixing-covid-vaccines-trial/story?id=75668099
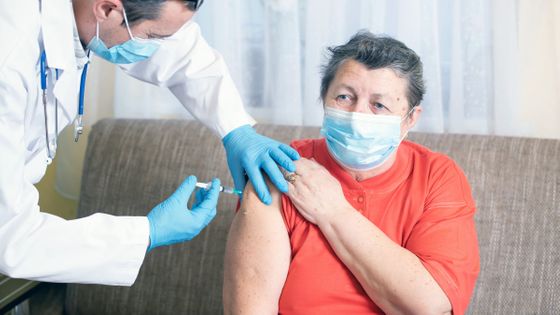
Vaccines for the new coronavirus need to be given multiple times at intervals, but due to the low availability of vaccines, it remains difficult to administer the same type of vaccine multiple times over the desired period. Therefore, the British government has invested 7 million pounds (about 1 billion yen) to start a study to confirm the efficacy of a combination of Pfizer BioNTech vaccine and Oxford University AstraZeneca vaccine. ..
The Pfizer BioNTech vaccine, which was the subject of this study, injects mRNA that has information on synthesizing the spike protein protruding from the surface of the new coronavirus, and synthesizes the spike protein in the patient's body. After that, the patient's body produces antibodies against the spike protein to acquire immunity to the new coronavirus.
What happens when you get the new coronavirus vaccine? --GIGAZINE
In addition, the Oxford University AstraZeneca vaccine is a type of vaccine called a 'viral vector vaccine' that transports the spike protein of the new coronavirus to a 'viral vector' that removes the pathogenicity from the virus. The patient's body then produces antibodies that give it immunity to the new coronavirus.
In this way, both vaccines have different mechanisms for delivering the spike protein of the new coronavirus into the patient's body, but immunity is acquired by the same method of producing an antibody against the spike protein in the patient. For this reason, the British government says that the combination of the two can be expected to be highly effective against the new coronavirus. If the efficacy of different types of vaccines can be confirmed in two doses, it will be possible to administer another vaccine even if the same vaccine as the first dose cannot be secured at the time of the second dose. , The supply of vaccines will be flexible.
In addition, viral vector vaccines have the problem that the effectiveness of the second and subsequent vaccine administrations is reduced because antibodies against the viral vector are generated during the first administration. However, a combination of mRNA and viral vector vaccines can avoid this problem, says Ian Jones, a professor of virology at the
The United Kingdom National Health Service (NHS), which is in charge of the research, confirmed the efficacy of the Pfizer BioNTech vaccine and the Oxford University AstraZeneca vaccine by changing the combination and administration interval, and confirmed the efficacy in the summer of 2021. The research results will be released by the time. The NHS is looking for subjects to cooperate with the study.
Coronavirus (COVID-19) vaccine research studies --NHS
https://www.nhs.uk/conditions/coronavirus-covid-19/research/coronavirus-vaccine-research/

Related Posts:
in Science, Posted by log1o_hf