Applying gene editing technology, a new coronavirus test method with only 'test time up to 1/6 of PCR method' is developed
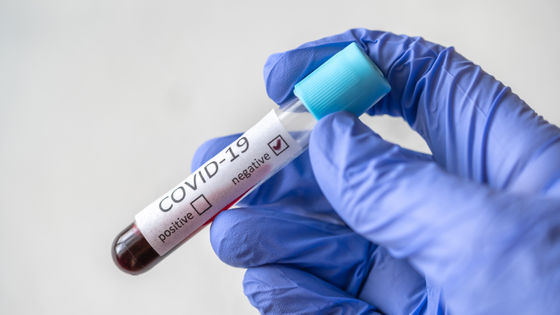
A new type of coronavirus testing method ' SARS-CoV-2 DETECTR ' has been developed that applies the gene editing technology '
CRISPR–Cas12-based detection of SARS-CoV-2 | Nature Biotechnology
https://www.nature.com/articles/s41587-020-0513-4
New CRISPR-Based COVID-19 Test Kit Can Diagnose Infection in Less Than an Hour | UC San Francisco
https://www.ucsf.edu/news/2020/04/417181/new-crispr-based-covid-19-test-kit-can-diagnose-infection-less-hour

KL.FM 96.7-News-Coronavirus: 'Rapid' test for COVID-19 'can show results in 45 minutes'
As of April 2020, the polymerase chain reaction (PCR method) , which is the mainstream of new coronavirus tests, usually takes 4 to 6 hours and has an accuracy of only 20 to 60%. is.
Meanwhile, the University of California, San Francisco (UCSF) announced in collaboration with San Francisco-based biotechnology company Mammoth Biosciences that it has developed a new new coronavirus test method, 'SARS-CoV-2 DETECTR'. According to UCSF, this is the first time that a new type of coronavirus test method using the gene editing technology CRISPR has been established.
UCSF's clinical medicine professor Charles Chiu's group of researchers focused on CRISPR because it can target any gene sequence. By using this technology, the research group is expected to detect two areas in the genome of the new coronavirus, 'a region commonly found in SARS coronavirus ' and 'a region unique to the new coronavirus' in parallel. We have succeeded in establishing an inspection method that can detect new coronavirus with high accuracy by adjusting the reagents.
One of the main advantages of the SARS-CoV-2 DETECTR is that it can be inspected quickly. Like the PCR method, the test itself uses a sample obtained by rubbing the mucous membrane of the respiratory tract with a cotton swab, but the PCR method takes 4 to 6 hours to produce results, compared to SARS-CoV-2 DETECTR The time required for the inspection can be greatly reduced to about 45 minutes.
Another feature of the SARS-CoV-2 DETECTR is that it can be introduced at a lower cost than the PCR method, which requires expensive specialized equipment, because it can use ready-made reagents and general equipment. In addition, the test result appears in a very clear form like 'commercial pregnancy test' indicating that the virus exists if a dark line appears, so the diagnosis of the new coronavirus can be made from the test result. It is easier to test than the PCR method, which requires a high degree of expertise to perform.
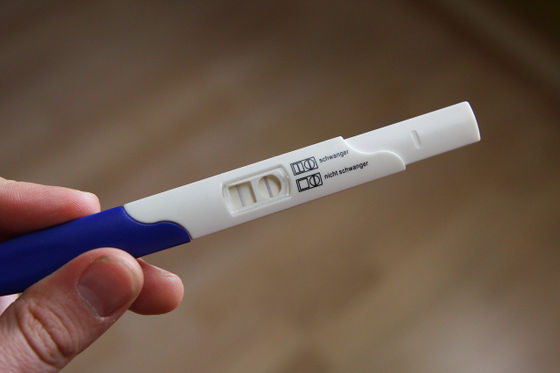
However, in order for this test method to demonstrate effective accuracy, it is necessary that the amount of virus in the sample be large, so the probability of overlooking a person at the early or late stage of infection when the new coronavirus in the body is relatively low is PCR. It is higher than the law. ' On the other hand, the research group says, 'Patients infected with the novel coronavirus usually have a very high viral load, so this defect has little impact on the test results.' According to the research group, the accuracy is 95% when the virus amount is sufficient.
The research group is currently conducting tests to obtain approval from the US Food and Drug Administration (FDA) , and once approved, SARS-CoV-2 DETECTR can be used at airports, schools, small medical institutions, etc. It is said that they are preparing.
Related Posts:
in Science, Posted by log1l_ks