Neuralink's electrode thread implanted in first patient's brain is only functioning 15% of the time, but FDA approves second-person clinical trial
Exclusive | Elon Musk's Neuralink Gets FDA Green Light for Second Patient, as First Describes His Emotional Journey - WSJ
https://www.wsj.com/tech/neuralink-gets-fda-green-light-for-second-patient-as-first-describes-his-emotional-journey-a2707584
Neuralink to implant 2nd human with brain chip as 85% of threads retract in 1st | Ars Technica
https://arstechnica.com/science/2024/05/neuralink-to-implant-2nd-human-with-brain-chip-as-75-of-threads-retract-in-1st/
Neuralink is developing a brain-computer interface (BCI) that connects the brain to a computer, aiming to improve the quality of life of paralyzed patients by implanting it in their brains. Neuralink's BCI is made up of a total of 1,024 electrodes attached to a total of 64 ultra-fine threads. These electrodes record the neural activity of nearby neurons and wirelessly transmit signals to operate a computer.
The first clinical trial to actually embed a Neuralink implant in a human brain was conducted in January 2024. You can watch the following article to see 29-year-old Noland Arbour, who underwent brain implant surgery, play chess on a PC just by thinking with his brain.
A movie of a paralyzed man who underwent Neuralink's human clinical trial 'operating a PC and playing chess just by thinking' is released - GIGAZINE
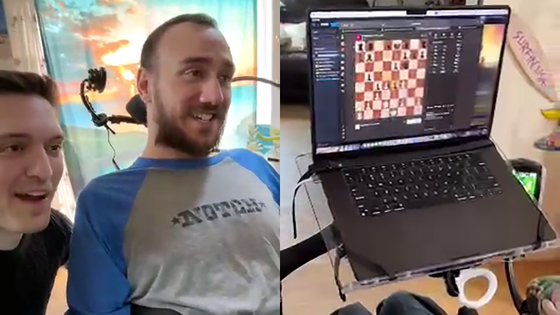
However, according to the Wall Street Journal, Arbour noticed that the device was no longer working properly a month after the surgery. This was because the electrode threads implanted in his brain had loosened and could no longer read the electrical signals that convert thoughts into cursor movements. In an interview with the Wall Street Journal, Arbour commented, 'It felt like I had fallen from a very high place to rock bottom. It was so hard, so hard, I cried.'
It has been reported that only 15% of the threads implanted in Arbour's brain are functioning properly, while the remaining 85% have shifted position and are no longer functioning properly. Because this was the first time such an implant had been implanted in a human brain, Neuralink was unable to accurately predict how much the implant would move inside the skull, and in fact it moved up to three times more than expected.
Arbour asked Neuralink if the implant could be removed or repaired, but the medical team refused to perform any further surgery and wanted to gather more information. 'I thought I had only scratched the surface of this amazing technology, and everything else was going to be taken away from me,' Arbour told The Wall Street Journal. 'But it only took me a few days to recover, and I realized that everything I'd done was for the benefit of those who came after me.'
In the end, the Neuralink team was able to improve the performance of Arber's brain implant by strengthening the algorithms and user interface. This gave Arber more capabilities than before the electrode threads came loose. 'I'm very hopeful about the future. We're learning a lot and things seem to be moving in the right direction,' Arber said.

The FDA also gave Neuralink approval to conduct a second human clinical trial, in which the company plans to implant longer 8mm threads instead of the 3mm to 5mm threads implanted in Arbour to fix the problem of the electrode threads coming loose.
Neuralink aims to have 10 patients implanted with brain implants by 2024 and plans to submit applications to Canadian and British regulators in the coming months.
Related Posts: