Earon Mask's brain implant company 'Neuralink' is planning an experiment targeting 'human beings'
Elon Musk's Brain Implant Company Is Inching Toward Human Trials - Bloomberg
https://www.bloomberg.com/news/articles/2022-01-20/elon-musk-s-brain-implant-company-is-inching-toward-human-trials
Elon Musk's brain implant company is inching toward human trials
https://techxplore.com/news/2022-01-elon-musk-brain-implant-company.html
Neuralink is a company that develops chips to be embedded in the human brain, and in August 2020, it announced the embedded chip `` Link '', and at this stage, Mr. Mask said, ``Link has already progressed to the stage where it can be tested in clinical trials. '' he said.
Neuralink CEO Elon Musk announces embedded chip ``Link'' & automatic surgery robot ``V2'' that connects the brain and AI - GIGAZINE

Also, although clinical trials on humans are not yet done, experiments to embed Link in the brain of monkeys have already been conducted, and images of ``monkeys playing games with only thinking'' have been released.
A video of a monkey with a chip embedded in the brain playing a game with only ``thinking'' will be released-GIGAZINE

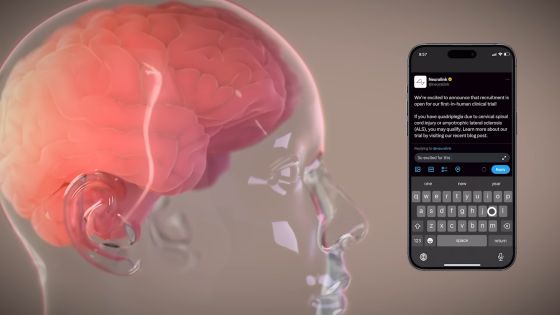
It has become clear that Neuralink is looking for a director in a clinical trial to embed its own chip in the human brain. According to the job posting, the director will lead 'the team responsible for enabling Neuralink's clinical research activities' and 'work closely with some of the most innovative physicians and top engineers to develop Neuralink's first clinical trial.' become a participant in the In addition, Mr. Mask told the Wall Street Journal, ``I hope to implant our device in some human brains in 2022.''
In order for a medical device manufacturer to obtain clinical trial approval from the US Food and Drug Administration (FDA), it must pass a test called a feasibility test. Based on the feasibility study, the FDA will then review the device and make a decision on whether to approve it.
It's unclear exactly where Neuralink is in this process. Bloomberg has requested comments from both Neuralink and the FDA, but no response has been received at the time of writing. However, 'generally, device makers will hire a clinical trial director early in their interaction with the FDA to design the trial to maximize their chances of obtaining approval from the FDA.' We speculate that Neuralink is in the early stages of the process of obtaining FDA approval.
Synchron, Neuralink's competitor, reports that it passed the FDA's feasibility test earlier.
Related Posts:
in Note, Posted by logu_ii