Neuralink, which develops a brain-computer interface to be implanted in Elon Musk's brain, begins recruiting subjects for its first human clinical trial

Neuralink , founded by Elon Musk, is a company that develops brain computer interfaces (BCI) that are implanted in the brain and communicate wirelessly with external devices. Neuralink is planning to conduct a human clinical trial using its independently developed BCI, and has begun recruiting subjects on its official website.
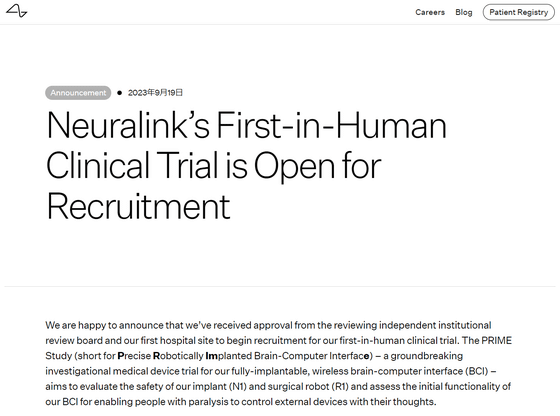
Neuralink's First-in-Human Clinical Trial is Open for Recruitment | Blog | Neuralink
https://neuralink.com/blog/first-clinical-trial-open-for-recruitment/
Neuralink clinical trial seeking human subjects | Mashable
https://mashable.com/article/neuralink-clinical-trials-recruiting-human-subjects
As of 2020, Neuralink has already succeeded in developing a chip to be implanted in the brain and a surgical robot that performs surgery to implant a chip in the brain. You can find out what kind of technology Neuralink's brain implant has by reading the article below.
Neuralink CEO Elon Musk announces embedded chip 'Link' that connects the brain and AI & automatic surgical robot 'V2' - GIGAZINE

Neuralink received approval to conduct human clinical trials in May 2023 from the U.S. Food and Drug Administration (FDA), the regulatory agency responsible for ensuring the safety and effectiveness of medical devices and products that emit electromagnetic waves. Masu.
Clinical trial application by Elon Musk's brain implant company 'Neuralink' finally approved by FDA - GIGAZINE
The reason why it took a long time for Neuralink to receive approval for human certification testing of brain implants is explained in the following article.
Why did it take so long for Elon Mask's brain implant company 'Neuralink' to receive approval for human clinical trials? -GIGAZINE

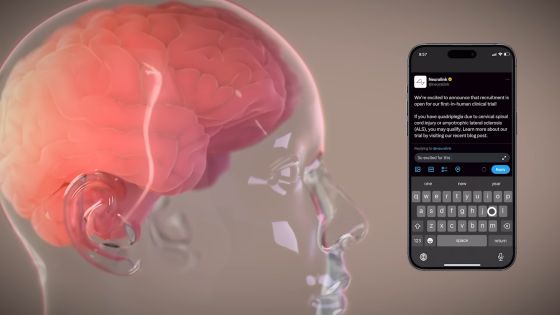
Neuralink is now recruiting participants for its first human clinical trial, which will be conducted at an independent institutional review board and Neuralink's first hospital facility. Neuralink's groundbreaking investigational medical device test using a fully implantable BCI seems to be named ``PRIME Study (Precise Robotically Implanted Brain-Computer Interface: Precise Robotically Implanted Brain-Computer Interface)''. The PRIME Study will be conducted to evaluate the safety of Neuralink brain implants (N1) and surgical robots (R1).
In the trial, N1 is surgically implanted into the brain using ultra-thin, flexible threads, in the area of the brain where R1 controls motor intention. Once N1 is implanted in the brain, it is 'completely invisible to the naked eye,' says Neuralink. The N1 is intended to record the implanted human's brain signals and transmit them wirelessly to an app that deciphers motor intentions. The initial goal of BCI is to allow people to control a computer's cursor or keyboard using just their thoughts.
Neuralink said, 'The PRIME Study builds on approval received from the FDA in May 2023 and is based on our commitment to create a generalized BCI to restore autonomy to people with unmet medical needs. 'This represents an important step in our mission.'
According to Neuralink, subjects in the PRIME Study are people with ``cervical spinal cord injury'' or ``quadrilateral paralysis due to amyotrophic lateral sclerosis (ALS).'' Subject registration is possible from below.
Patient Registry | Neuralink
https://neuralink.com/patient-registry/
Related Posts:
in Hardware, Posted by logu_ii