FDA officially approves Apple Watch's atrial fibrillation history feature as a medical device development (MDDT)

The Apple Watch can detect irregular heartbeats that indicate
FDA qualification of a new Medical Device Development Tool
https://content.govdelivery.com/accounts/USFDA/bulletins/399d551

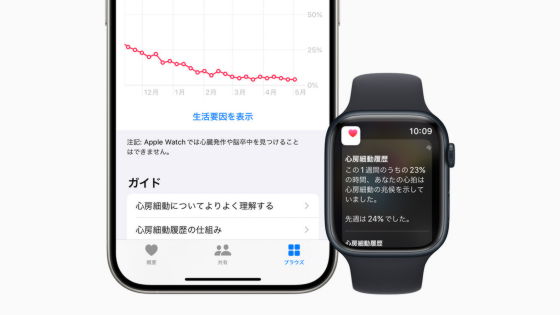
The Atrial Fibrillation History feature analyzes heart rate data collected by the optical pulse pulse (PPG) sensor on Apple Watch to provide users with an estimated atrial fibrillation (AFib) burden via the Health app on iPhone. You can track and trend your estimated atrial fibrillation burden over time and include data visualizations to understand how your lifestyle is impacting AFib.
Map File: AFIB
(PDF file) https://www.apple.com/legal/ifu/afhf/1-0/afhf-1-0-ja_JP.pdf
The FDA's MDDT program is an effort to approve tools for use by medical device sponsors in the development and evaluation of medical devices. The MDDT program promotes the use of tools such as biomarker tests, clinician outcome measures, computer models, and digital healthcare technologies such as sensors and wearables.
Because tools approved for MDDT play a critical role in understanding the safety, effectiveness, and other performance aspects of a medical device, the FDA evaluates the tool and supporting evidence through a rigorous review process to determine whether they produce scientifically valid measurements for their intended use and context.
'Apple Watch's atrial fibrillation history feature is the first digital healthcare technology authorized under the MDDT program and provides a non-invasive method of obtaining estimates of atrial fibrillation burden within clinical studies,' the FDA said in a statement. 'It is specifically designed for use in clinical studies evaluating the safety and effectiveness of ablation therapies and devices intended to treat atrial fibrillation.'

The atrial fibrillation history feature is only available in some regions and is not available in Japan at the time of writing, but the notification function for irregular heartbeats suspected to be a sign of atrial fibrillation is available from watchOS 7.3, released in November 2021.
Apple Watch introduces ECG app and irregular heart rate alerts - Apple (UK)
https://www.apple.com/jp/newsroom/2021/01/ecg-app-and-irregular-rhythm-notification-coming-to-apple-watch/

Related Posts:
in Hardware, Posted by log1i_yk