How is the new coronavirus vaccine made?

How Pfizer Makes Its Covid-19 Vaccine --The New York Times
https://www.nytimes.com/interactive/2021/health/pfizer-coronavirus-vaccine.html
◆ DNA amplification
The Covid-19 vaccine produced by Pfizer BioNTech is a vaccine called 'mRNA vaccine ' that induces an immune response using mRNA transcribed from artificially amplified 'DNA that produces the spike protein of the new coronavirus'. ..
Pfizer's facility in Chesterfield, Missouri, stores circular DNA called plasmids that contain peplomer DNA. Incubating E. coli into which this plasmid has been introduced for 4 days produces trillions of copies of the plasmid.
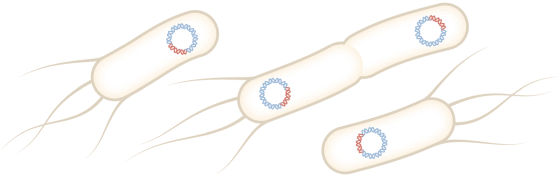
After culturing for 4 days, only the plasmid is removed by adding a treatment to destroy E. coli. After that, it is
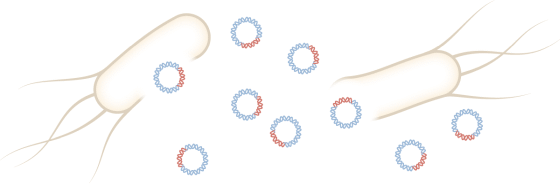
The
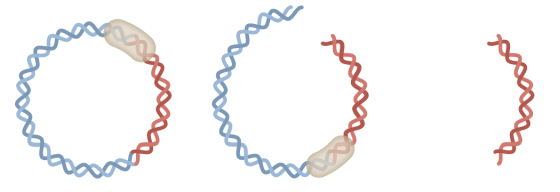
In addition, it is said that 1.5 million COVID-19 vaccines can be made per 1 liter bottle.
◆ Transcription to mRNA
At Andover's facility, five bottles of DNA are thawed over a day, mixed with the enzymes and mRNA components needed for transcription into mRNA, and transcribed for several hours.
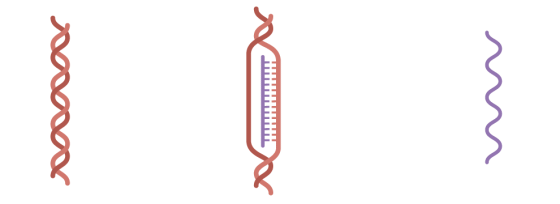
The transcribed mRNA is sent to a facility in Kalamazoo , Michigan, at -20 degrees Celsius for processing as a vaccine after removing unnecessary DNA, enzymes, impurities, etc. Samples will also be sent to the Chesterfield facility to confirm the sequence.
◆ Processing into vaccine
At the Kalamazoo facility, mRNA is thawed, mixed with water, and then mixed with lipids to facilitate entry into human cells to produce lipid nanoparticles. Special equipment using microfluidics is required to generate these lipid nanoparticles.

After the lipid nanoparticles are completed, impurities are removed and sterilized, and then the vaccine is sealed in a

After encapsulation in the vial, the vaccine is cooled to minus 70 degrees Celsius and quality tested at the Chesterfield and Andover facilities for four weeks. Pfizer runs a 60-day process from DNA production to vaccine completion, of which more than half is spent on quality testing.
◆ Correspondence to mutant strains
With the spread of the new coronavirus infection, the existence of mutant strains for which existing vaccines are not effective has been reported. According to the New York Times, Pfizer and BioNTech are developing a new version of the vaccine for the mutant strain, which could soon mass-produce a vaccine that is effective against a particular mutant strain.
Related Posts:
in Science, Posted by log1o_hf