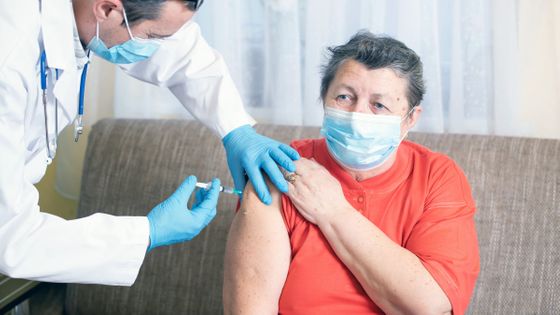
The U.S. government announced that it would not change its plan to vaccinate twice, and argued that 'many people should be vaccinated once.'
In principle, Pfizer and Moderna's new coronavirus vaccines, which have begun to be inoculated in the United States and the United Kingdom, are inoculated twice with a certain period of time. However, due to concerns about a relapse of the pandemic and the unexpected delay in the spread of the vaccine, the opinion that 'the vaccine dose should be halved and more people should be vaccinated'. Is also out. In response to this opinion, the US Food and Drug Administration (FDA) issued a statement on January 4, 2021 that 'there are no plans to change the prescribed vaccination schedule.'
FDA Statement on Following the Authorized Dosing Schedules for COVID-19 Vaccines | FDA
https://www.fda.gov/news-events/press-announcements/fda-statement-following-authorized-dosing-schedules-covid-19-vaccines
FDA tells US health providers not to modify COVID-19 vaccine dose schedule --The Verge
https://www.theverge.com/2021/1/5/22214865/covid-vaccine-schedule-doses-fda-uk
The new coronavirus vaccine ' BNT162b2 ' jointly developed by Pfizer and BioNTech and the moderna 'mRNA-1273 ' are said to need to be given twice, 21 days and 28 days apart from the first vaccination, respectively. I am.
However, governments in several countries have decided to change their vaccination schedules due to the failure to secure vaccines and delays in vaccination plans. Danish government on January 4, 2021, as 'a period of up to the second vaccination, an admission that delayed until after six weeks at the maximum from the first vaccination' announcement was. This measure is intended for both Pfizer's vaccine, which is given at 3-week intervals, and Moderna's vaccine, which is given at 4-week intervals.
Following the Danish government's decision, Germany and Ireland have made similar moves, and the United Kingdom has approved a 'up to 12 weeks' interval.
Vaccine developers have expressed opposition to plans to delay the second vaccination beyond the prescribed procedure. Pfizer and BioNTech released a statement on January 4 that 'the safety and efficacy of vaccines at different times of vaccination have not been verified.' He emphasized that 'there is no evidence that there is a preventive effect even if the interval is longer than the prescribed period.'
In response to these discussions, the FDA issued a statement on January 4 that it would 'adhere to the approved dosing schedule,' clearly stating that it had no plans to change the prescribed vaccination procedures. ..
In a statement, FDA Secretary Steven Hahn and Director of the Center for Biopharmacy Evaluation and Research, Peter Marks, said, 'Discussion on changes in dosing schedules or doses will do so,'Vaccines faster and more. We know that it is based on the belief that it will be possible to reach people, but plan changes that are not supported by proper scientific evidence will ultimately go against public health. It may be effective. Until the vaccine manufacturer obtains scientific data to support the plan change, it is strongly recommended that healthcare providers continue to inoculate each new coronavirus vaccine according to the FDA-approved dosing schedule. I will. '
In a statement from foreign media, The Verge said, 'Delaying a second vaccination may result in only partial immunity, and if a person who has been vaccinated only once becomes infected with the virus. There is also a risk that the surviving virus will mutate, 'suggesting the risk of the new corona virus becoming resistant to the vaccine.
Experts have also said that changing the vaccination plan due to the change of the morning decree will damage the trust of the people. Natalie Dean, an assistant professor of medical statistics at the University of Florida, told Twitter: 'I will continue to support a consistent position for the foreseeable future in changing vaccination strategies at the last minute. We should not underestimate the importance of public confidence in the decision-making process for success, 'he said in a negative view of the plan change.
Regarding last-minute changes to vaccination strategy, I'm going to keep beating the same drum for a while. Please don't underestimate the importance of the public having * trust in the process * for long-term success. Social scientists --we need you! https://t.co/IeiDZty4IN
— Natalie E. Dean, PhD (@nataliexdean) January 4, 2021
Related Posts:
in Note, Posted by log1l_ks