How does it freeze like freezing soap bubbles?

Soap bubbles are made of liquid such as soapy water, so of course they will freeze in a cold environment. It is known that the appearance of such soap bubbles freezing is a little different from the appearance of ordinary water freezing.
How soap bubbles freeze | Nature Communications
https://www.nature.com/articles/s41467-019-10021-6
Bubble of an idea leads to new research on freezing | Virginia Tech Daily | Virginia Tech
https://vtnews.vt.edu/articles/2019/06/me-freezingbubbles.html
A research team led by Associate Professor Jonathan Boriko of Virginia Tech's Faculty of Mechanical Engineering said he was wondering why the ice crystals floating around the soap bubbles were floating when he saw the movie where the soap bubbles freeze. .. The following movie is a picture of a soap bubble freezing.
Bubbles Freezing in Slow Motion-YouTube
When you blow soap bubbles on the snow ...

A vortex winds over the soap bubble film, and you can see ice fragments shaped like stars flying in the vortex.

The ice pieces get bigger and bigger and slow down.

Ice grows like fern leaves from the lower hemisphere ...

If you think the whole soap bubble is covered with ice ...

It popped with a click.

And you can see the state of the experiment that Associate Professor Boriko actually frozen the soap bubbles from the following movie.
If you place the soap bubbles on a substrate that has been cooled to below freezing at room temperature, the soap bubbles will begin to freeze from the grounded lower hemisphere.

The boundary between ice and liquid that rises with wrinkles is called the 'freezing front.'
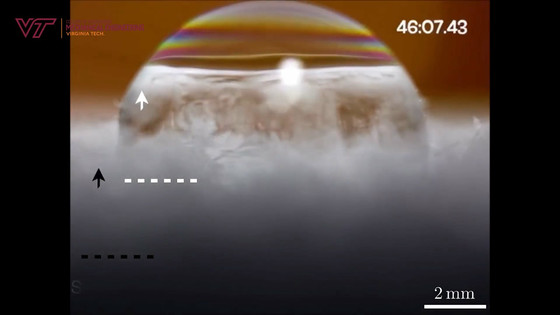
And before the whole thing froze, the upper third collapsed and sank like a deflation.

Next, when I put the soap bubbles on the board of -20 degrees in the room of -20 degrees, the soap bubbles froze in an instant.

According to Associate Professor Boriko, when soap bubbles are placed on a substrate at -20 degrees Celsius at room temperature of -20 degrees Celsius, freezing creates a temperature gradient and the temperature at the bottom of the foam rises above the rest of the foam. That thing. As a result of the temperature gradient, the temperature at the top of the soap bubble is -20 degrees, while the temperature at the bottom is -6 degrees. This temperature gradient creates a flow called
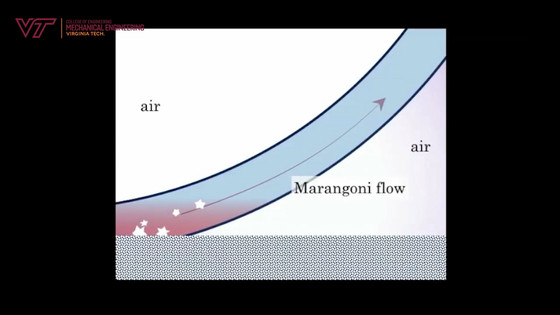
Therefore, when you capture the appearance until it freezes, the frozen front does not gradually advance, but the frozen part flows over the soap bubble film ...


It looks like the ice wraps around the entire soap bubble. The reason why the frozen soap bubbles broke in the first movie is thought to be that the surface tension is reduced by the Marangoni convection.

Associate Professor Boriko said, 'In the past, it was thought that how fast a freezing front grew would determine how long the object would freeze. However, Marangoni convection created hundreds of freezing fronts at the same time. That's why. ' It became clear that instead of slowly freezing, the whole thing would freeze at once.
Related Posts: