The existence of a 'penta diamond' that is even harder than that diamond is predicted

Not only popular as a gemstone since ancient times, but also used in industrial cutters and scientific experiments,
Phys. Rev. Lett. 125, 016001 (2020)-Pentadiamond: A Hard Carbon Allotrope of a Pentagonal Network of sp 2 and sp 3 C Atoms
https://journals.aps.org/prl/abstract/10.1103/PhysRevLett.125.016001
Researchers building a harder diamond, called pentadiamonds
https://phys.org/news/2020-07-harder-diamond-pentadiamonds.html
University of Tsukuba|Notice/Information|Remarkable Research|Predicting the existence of carbon crystals that are lighter and tougher than diamond ~Penta diamond promotes new development of material science~
http://www.tsukuba.ac.jp/attention-research/p202007011400.html
Boasting one of the highest hardness among natural substances, diamond is produced when carbon is placed under high temperature and high pressure conditions in the ground. The carbon atoms that make up diamond can be covalently linked to adjacent atoms, and carbon is crystallized by the covalent bonds between carbon atoms.
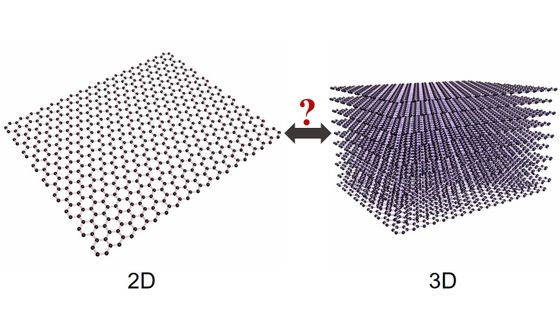
The hardness of a substance depends on how the substance is bound, and how carbon is covalently bound is greatly influenced by the ' atomic orbital ' of how the electron of a carbon atom behaves. .. Usually of diamond is ' sp3 hybrid orbital is crystal born covalently bonded in,' but, the research team of the University of Tsukuba 'sp3 hybrid orbital and sp2 hybrid orbital was investigated what would happen if that was crystallized by a combination of'. 'The allotrope containing carbon atoms in the sp2 hybrid orbital and sp3 hybrid orbital has a large morphological diversity depending on the combination and position of the bonds '.
Based on the density functional theory , the research team calculated the electronic state of carbon by computer and calculated the most stable atomic configuration. As a result, the research team predicted that the pentagonal crystal “ pentadiamond ” has a hardness higher than that of conventional diamond.
The figure below shows the crystal structure of conventional diamond. The feature is that carbon atoms are connected to each other by four covalent bonds.
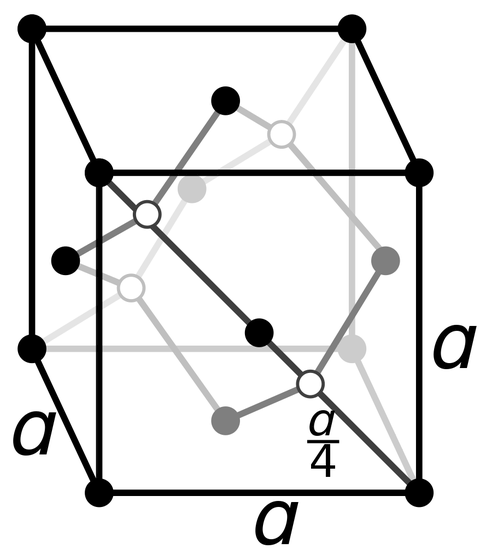
by Viktor Håkansson Ingre
And the following figure is the crystal structure of pentadiamond. You can see that the structure is like a combination of pentagons with 5 carbon atoms.
The pentadiamond is a result of simulation to the last, not actually synthesized. Still, according to the simulation by the research team, pentadiamond exceeds Young's modulus and rigidity, which indicate the hardness of the material, compared with conventional diamond. In addition, the hardness of the conventional diamond is about 1200 giga Pascals whereas it is, penta diamond seems to have been calculated to be proud of the hardness of 1691 Giga Pascal. 'Pentadiamond is not only harder than conventional diamond, but also has a density as low as graphite of the same carbon allotrope,' explains Assistant Professor Mina Maruyama of the Nanostructures Laboratory.
In addition, Professor Shin Okada of the Nano-Structure Physics Laboratory said, 'With this pentadiamond, there is a possibility that the material can be fundamentally redesigned. In addition to industrial applications such as cutters and drills, There is a possibility that pentadiamond can be applied to a diamond anvil cell that can reproduce extreme pressures.'
Related Posts:
in Science, Posted by log1i_yk