DARPA has started to develop a `` preventive drug for new type coronavirus infection '' that can be mass-produced

The
US Military Scientists Hope To Have Coronavirus Therapeutic By Summer-Defense One
https://www.defenseone.com/technology/2020/03/us-military-scientists-hope-have-coronavirus-therapeutic-summer/163659/
DARPA Races To Create a “Firebreak” Treatment for the Coronavirus-IEEE Spectrum
https://spectrum.ieee.org/the-human-os/biomedical/bionics/darpas-firebreak-treatment-for-the-coronavirus
This prophylactic drug utilizes the mechanism that produces antibodies against the new coronavirus in patients who have already completely cured COVID-19. First, a blood sample is collected from a patient who has completely cured COVID-19, and all protective antibodies produced by B cells in the body are extracted. Next, we use bioinformatics to examine all protective antibodies, find the ones that are most likely to be effective against the new coronavirus, and identify the messenger RNA of the new coronavirus that stimulated the production of that antibody.
When this messenger RNA is injected into a healthy human body, antibodies against the new coronavirus are temporarily produced. The phenomenon of antibody production in the body by preventive drugs is expected to last for several months, and the research team asserts that the effect is 'it is effective in preventing COVID-19, sometimes even as a therapeutic drug.' 'It is cheaper and easier to have the human body produce the antibody than to produce the antibody itself in a
This development process is based on the Pandemic Preparedness Platform (P3), a pandemic preventive drug development program launched by DARPA in 2017. The P3 program 'establishes research methods for viruses that can cause pandemics that hinder military purposes.' Previously it took years using new technologies in DNA sequencing, such as investing in microfluidics , manipulating sub-millimeter liquids, nanofabrication , and producing objects under one billionth of a meter. With the track record of shortening the discovery process for monoclonal antibodies to a few weeks, the new coronavirus has been put to practical use.
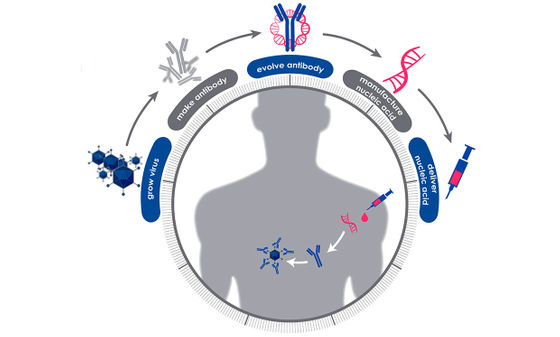
As of March 5, 2020, the research team reports that it is 'in the process of examining all protective antibodies extracted from blood samples collected from dozens of patients who have completely cured COVID-19.' 'It takes at least 90 days to identify the COVID-19 messenger RNA that drives antibody production,' said Dr. Amy Jenkins, head of the research team. As for the completion of the prophylactic drug prototype, 'if it takes shape by the end of summer,' he said.
However, even if a prophylactic drug is completed, it will need to undergo clinical trials for safety and obtain approval from the U.S. Food and Drug Administration (FDA), so 'it will take more time before it can be used actually.' There are some points. Responding to this concern, the research team said, 'Using a system that allows the use of unapproved drugs in limited situations, such as when there is no alternative In this case, COVID-19 is a life-threatening infectious disease, and the preventive drugs under development are considered to be mechanically safe, so compassionate use is applied. I am convinced. '
Related Posts:
in Note, Posted by darkhorse_log