Scientists develop efficient ways to extract oxygen from lunar regolith

by
In recent years, the National Aeronautics and Space Administration (NASA) has announced a “Artemis Project” that will send humans back to the moon by 2024. The lunar surface is not a suitable environment for human survival, but researchers are exploring ways to improve the lunar environment in a variety of ways, saying, `` Efficiently removing oxygen from lunar deposits (regolith) Scientists have developed a method of extraction.
Proving the viability of an electrochemical process for the simultaneous extraction of oxygen and production of metal alloys from lunar regolith-ScienceDirect
https://www.sciencedirect.com/science/article/abs/pii/S0032063319301758
Scientists Have Figured Out How to Extract Oxygen From Moon Dirt
https://www.sciencealert.com/scientists-have-figured-out-how-to-extract-oxygen-from-moon-dirt
Oxygen is indispensable for human life, and it is easy to think that the moon has no oxygen. However, since the regolith that covers the surface of the moon actually contains many oxygen atoms, extracting oxygen atoms from the regolith may make it easier for people to live.
From the results of analyzing regolith samples collected on the moon, it has been found that 40 to 45% of the weight of regolith is oxygen atoms, but there is a big problem in using oxygen atoms . Bethany Lomax , a chemist at the University of Glasgow , pointed out, “Oxygen is a very valuable resource, but it cannot be used immediately because it is chemically bound to minerals and glass as an oxide.” It is.
Therefore, Lomax and his team developed a method to extract oxygen atoms from regolith and conducted experiments. Although the actual regolith sample cannot be used for the experiment, the ' simulated regolith ' that reproduces the scientific characteristics and particle size of the analyzed regolith with materials on the ground was used for the experiment.

by Wikimedia Commons
In previous approaches to extracting oxygen from simulated regolith, a technique was used in which hydrogen atoms were used to reduce iron oxide to produce water, and then electrolyzed water to separate oxygen. However, these techniques are not practical because the process is complicated and the efficiency is poor, or when increasing the efficiency, it is necessary to heat the regolith to such a high temperature that it melts.
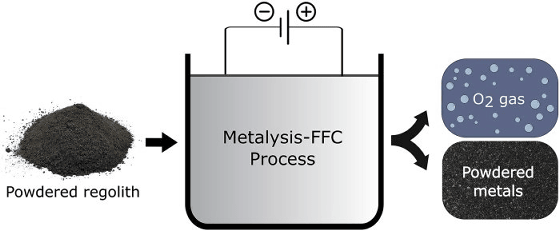
The research team eliminated the process of generating water by chemical reduction and adopted a method of directly electrolyzing the powdered simulated regolith. “Oxygen extraction was performed using a technique called
Lomax et al. First put simulated regolith powder in a mesh-lined basket, added calcium chloride as an electrolyte, and heated the mixture to 950 degrees Celsius. When current is passed, electrolysis occurs and oxygen is extracted.
by Lomax et al
As a result of the experiment, 75% oxygen was extracted in the first 15 hours, and 96% oxygen was successfully extracted from the simulated regolith powder in about 50 hours. One-third of the extracted oxygen was detected in a reusable state, and the rest was lost due to corrosion of the reaction vessel, but it was still successful in extracting oxygen with higher efficiency than conventional methods. The In addition, it seems that the problem of losing the extracted oxygen due to corrosion can be solved by improving the method.
As a result of the oxygen has been removed from the simulated regolith powder remaining material is an alloy that the generated as it is possible to reuse the alloy for another purpose. `` This process gives the moon pioneer access to the fuel and oxygen needed for life support, as well as a wide range of alloys for on-site manufacturing, '' said James Carpenter, head of moon strategy for the European Space Agency (ESA). Can also access '.

Related Posts:
in Science, Posted by log1h_ik