Why is 'reverse-innovation' where many people saved with cheap medical tools not happening in medical care?
by sasint
Contrary to the conventional method of transferring technologies and products from developed countries to emerging countries, the relocation of technologies and products born in emerging countries to developed countries is called " reverse innovation ". Tom Vanderbilt , a journalist, says that there are many high walls behind the scenario of reverse-innovation in the medical field that is less prone to occur with cheap tools.
"Reverse Innovation" Could Save Lives. Why Are not We Embracing It? | The New Yorker
https://www.newyorker.com/science/elements/reverse-innovation-could-save-lives-why-isnt-western-medicine-embracing-it
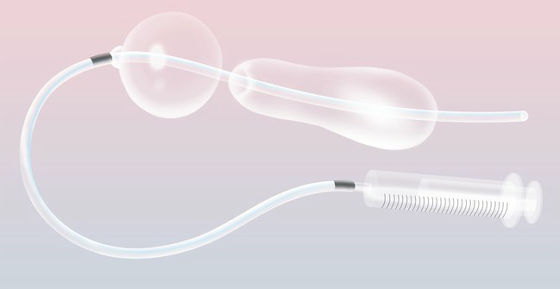
More than half of maternal deaths in developing countries, and one-third of all global maternal deaths are due to postpartum bleeding (PPH). Postpartum hemorrhage is a symptom that the amount of bleeding can not be controlled due to uterine relaxation etc. after birth, etc. Although it is dealt with by methods such as administration of uterine contraction drug and hysterectomy, in South Sudan where supplies are lacking, such a method It was not collected. So Burke Doctor and his colleagues who worked in South Sudan adopted a tool called "Uterus Balloon." The uterine balloon squeezes the bleeding spot and has the power to help the body's blood coagulation reaction.
However, because the standard uterine balloon was expensive for about $ 300 (about 33,000 yen), doctors Burke and others are cheap Uterine Balloon Tamponade (Uterus Balloon · Tamponade / UBT) which is not subject to regulation, I began to pay attention to the idea of. UBT was invented in 2000 by Bangladeshi doctor Sayeba Akhter and is made from standard catheters and condoms. Dr. Burke thought that it would be impossible to create a tool between the medical standard womb balloon and the DIY UBT.
And it was developed " ESM-UBT " which added a valve so that the condom swells properly and packaged as one item. ESM-UBT is a kit formula that makes it easy to replace parts, and making it possible to understand how to use it with a picture even if it can not read the letters is used in many countries from Peru to Zambia I came to be caught. The survival rate of women who developed PPH in these countries by ESM-UBT seems to have increased to 97%. In addition, it is said that medical cost has also decreased due to the reduction in the number of hysterectomy surgery and blood transfusion. Because Burke doctors did not take patents on their own developed technology, it is also admired that anyone can freely use ESM-UBT.
The idea of Burke is a bottom-up approach that started from the scene in a general society that top-down is the top down. Such tools are, of course, not ideal. But on the other hand, Burke noted that introducing the latest technology in an environment without adequate supply chain or technical support could be an exceptional action.
Doctor Burke does not wait for expensive technologies to be cheaper or have aid from relief agencies, observe what actually is used at the work site, It means "to produce the best policy". Although CPAP equipment to assist breathing is standard in the United States, it is too expensive in emerging countries, so many doctors working in emerging economies like "connecting tubes to a bottle of cola with water in it" , I will take a solution to the situation. While this method increases the survival rate of newborn babies, it raises the risk of blindness. So Burke created ESM - cpap with a similar approach to ESM - UBT to solve the problem. Although it is not high-tech, ESM-cpap costs about $ 30 (about 3300 yen).
We call reverse innovation to introduce technological innovation (innovation) born in emerging countries into developed countries and disseminate it to the world. One well-known reverse innovation has orthopedic fluid therapy (ORT). ORT was noted in Bangladesh in 1968 as a treatment for dehydration symptoms associated with cholera. There was a movement to introduce ORT even in industrialized countries that were administered to infusion as a treatment for diarrhea, but this approach was to be resisted by the doctor. One reason for this is that infusion brings many benefits.
by stux
Reverse innovation is a valuable tool to improve performance and lower medical costs for many people, but there are many challenges to spread in developed countries.
One of the challenges lies in the fact that the idea of cultural bias, that is, what can we learn from learning from a country like sending experts from developed countries? Also, there is a problem that most hospitals look skeptical when someone presents "cheap and good way" in the presence of a competitive market like the United States. And even if "cheap and good way" gets approval from the US Food and Drug Administration (FDA) at a high cost, organizations dealing with inexpensive tools are "expensive for physicians who are not interested in cheap solutions" Manufacturers trying to sell products "must fight.
Of course, despite the existence of many high hurds, there are people who are trying to bring about reverse innovation. However, " destructive innovation " that Netflix destroyed the rental video market has hardly happened in the healthcare market.
Meanwhile, the fact that recruitment of reverse-innovation medicine by non-wealthy people in the United States treats low-income countries and people of the United States in the same way, also points out that it is "a form of new discrimination" . Also, Professor Michael Saag of the University of Alabama, Birmingham explains, "Even in Sub - Saharan Africa or in the countryside of Alabama Province, the condition of care that pregnant women can access is the same", but I will explain to Nancy Kass of Johns Hopkins University The professor states that "many of the Americans refuse to recognize that they are" inferior to standard medical care "even without other methods."

by truthseeker08
Dr. Birk's womb uterus balloon has attracted the attention of healthcare workers in the United States and Canada and is under review by the FDA at the time of writing the article. However, even if FDA approval is obtained, it is said that it is not expected to become available in the United States shortly due to the reasons mentioned above.
Related Posts:
in Note, Posted by darkhorse_log