Genetic engineering is used to protect horseshoe crabs with "blue blood" that saves human life

ByCJ Oliver
Living fossils that have lived on Earth for hundreds of millions of years without changing their appearanceHorseshoe crab"Blue blood" flowing greatly differs from other animals in the body of the body. Because this blood reacts extremely sensitively to toxins, it is used as a raw material for reagents that detect toxins, but horseshoe crabs are due to the fact that the number of individuals is drastically decreasing and therefore blood is collected from the living body The opposite voice is also rising. Meanwhile, utilization of alternative reagents that have been studied since the 1980s is about to spread.
The Last Days of the Blue - Blood Harvest - The Atlantic
https://www.theatlantic.com/science/archive/2018/05/blood-in-the-water/559229/
It is known that horseshoe crabs inhabited the earth from a very old age and fossils were found from the strata of 450 million years ago. So far on EarthMass extinction of creatures that have occurred many timesSurviving and having a different aspect from the present earth "Pangea super continentIt is the only creature on the earth who has seen all the era when it emerges and disappears.
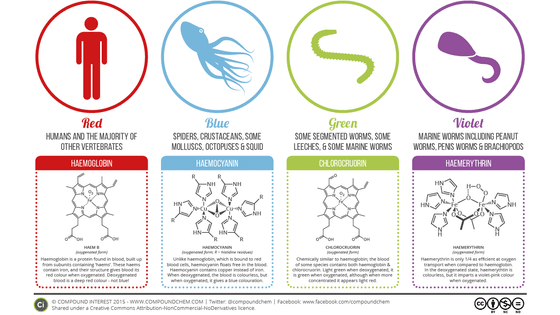
Such a horseshoe crab body has a pale blue colored blood flowing. The red blood of a human-like creature is red because iron is contained in the oxygen-carrying substance "hemoglobin", but since the blood of horseshoe crabs contains copper instead of iron, the blue color doing. And it has long been known that this blood reacts extremely sensitively to the toxin "endotoxin" and it acts to solidify and confine the toxin. Its sensitivity is so high that it reacts even when the toxin is contained at 1 ppt (1 trillionth) level.
This property is utilized to save the life of mankind. Especially at the medical site, it is indispensable to thoroughly eliminate toxins, and the "LAL test" using the LAL (horse horsetail extract component) reagent collected from the blood of horseshoe crabs was conducted to confirm safety It is.
LAL basic knowledge | Wako LAL system
http://www.wako-chem.co.jp/lal/lal_knowledge/index.html
This LAL reagent is produced by separating substances that cause a clotting reaction from horseshoe crab blood, but the material blood is collected from living limulus. The horseshoe crabs of over 400,000 animals per year are captured, and the process of collecting about 30% of the blood and returning it to the original sea is repeated, but there are a certain number of individuals who inevitably die, the number It is said that 50,000 animals are. In recent years, since the number of horseshoe crabs has been confirmed to dramatically decrease the number of individuals, the necessity of developing LAL reagents not dependent on the living body of the horseshoe crab is increasing.

Biologist Jeak Ling Ding is one of the researchers who spent many years in researching such alternative LAL. Ding said that he was searching for mounds in search of horseshoe crabs since the 1980's, along with Bow Ho, a husband and research partner, have been pursuing ways to make LAL reagents that do not use living limulus.
To that end, Ding et al. Focused on generating DNA by manipulating microorganisms that could produce the necessary substances. It is known that the blood of horseshoe crabs contains specific molecules called "Factor C" that react strongly to toxins, Mr. Ding extracts the DNA part concerning the creation of this substance, and in the laboratory We proceeded research on a method of joining it to DNA of cells of microorganisms such as "yeast" that can be easily cultured. This method had already been put into practical use in an attempt to produce "insulin" necessary for treating diabetes in genetically modified Escherichia coli.
As a result of the research, Mr. Ding and Mr. Ho succeeded in identifying genes related to Factor C, and in the yeast DNA(PDF)Successful incorporationDid. And, although they were both actually observing whether factor C is secreted from yeast, the first trie failed. After that, another method using yeast cells and mammalian cells was tried, but it seems that the period of failure has continued in either case.
A turning point came in the latter half of the 1990s. Mr. Ding and Mr. Ho in the US gave a lecture on "baculovirus vector system" with other cells as the host, it became a big hint to advance the research. With this technique, you can use it as a "culture plant" of factor C by incorporating the factor C gene into insect internal organ cells using the virus. Although the horseshoe crabs contain "crab" in their name, they are not actually crustaceans like crabs, but the same as spiders and scorpionsScissors typeIt belongs to creatures. Furthermore, both insects and horseshoe crabsArthropodaIt belongs to the same ancestry because it belongs to the same ancestry, so it is compatible with each other and this attempt surprisingly goes well and has good results.

ByKayLambPhotos
Thus, 15 years after the start of the research, Mr. Ding and Mr. Ho finally succeeded in establishing a method to produce an alternative reagent which substitutes LAL reagent without using horseshoe crab.
But the situation did not change soon. In 2003, an inspection kit using substitution reagent based on Ding's patent was launched, but even few pharmaceutical companies are interested in it, few LAL reagents taken from the horseshoe crab still in the world continue to be used There is a situation that it is.
It is a fact that there were certain reasons for such a situation. We are supplying the inspection kit by the new technology "Lonza(Lonza) "Because it was only one company, the pharmaceutical company hated relying on a single supplier considering the risk of the supply of the product being disrupted. Also, it was one of the reasons that there was resistance from companies that bleeding and selling from horseshoppers. There are six companies that collect blood of horseshoe crabs in the United States, two of which refuse to interview The Atlantic and no response from one company. The two companies have practically no external window, and the last one is Lonza, which manufactures alternative reagents. In other words, Lonza is a manufacturer handling both horseshoe crab blood and alternative reagents.

ByHealth Gauge
Ding said that he was disappointed in the face of this business reality. Looking back then, Mr. Ding said, "We were happy to be purely researching as a scientist, we were happy.We said that if Factor C replacement products spread all over the world and horseshoe crabs are saved I thought that. "
A tough time has continued for alternative reagents, but in recent years the situation has changed. In the field of alternative reagents, which was the exclusive supply of Lonza, "hygros" entered as a substitute factor C supplier for the second company in 2013, and significant progress has been made in terms of stability of supply. Kevin Williams, a senior scientist at Highgloss, says that this step is a "long-postponed modernization" step. In the past, pigs have been used for the production of insulin, which is necessary for the treatment of diabetes, but in the past few decades their production has been replaced with yeasts and bacteria, the use of pigs is becoming a thing of the past. That same movement is happening even in the situation surrounding LAL and horseshoe crab.
In terms of legal restrictions, a situation is being born that will encourage this. In 2016, the use of an alternative factor C to make alternative reagents in Europe is permitted, and the US is also expected to follow. By lowering barriers in both supply and regulation, we are trying to prepare an environment for producing reagents important to humans without threatening the precious life of horseshoe crabs.

ByAngel Schatz
Related Posts: