New discovery that rechargeable and safe lithium batteries become realistic

ByRob Nunn
Batteries with higher energy storage capacity than "Li-ion batteries" used in notebook PCs and smartphonesLithium battery"Has not been widely used so far because it has a problem of safety due to short circuiting when made in a chargeable form. But,Cornell UniversityIt seems that the research team finally cleared up this problem.
Room-temperature lithium metal battery closer to reality | Cornell Chronicle
http://news.cornell.edu/stories/2016/02/room-temperature-lithium-metal-battery-closer-reality
Nanomembrane May Bring Rechargeable Lithium-Metal Batteries Back - IEEE Spectrum
http://spectrum.ieee.org/nanoclast/semiconductors/materials/nanomembrane-may-bring-rechargeable-lithiummetal-batteries-back
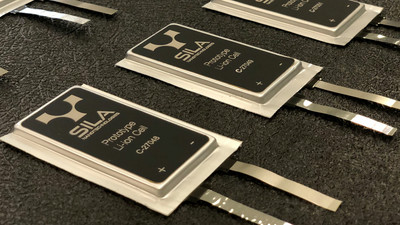
A lithium metal oxide, A secondary battery (rechargeable battery) using carbon which occludes lithium in the negative electrode is "Lithium ion battery"It is widely used for notebook PCs, smart phones, hybrid cars, airplanes, etc. on the other handMetallic lithium, And the use of fluorinated graphite and thionyl chloride for the positive electrode is "Lithium battery"is.
When lithium is repeatedly charged, the lithium is interposed between the positive electrode and the negative electrodeDendrite (dendritic crystal)There is a danger of short-circuiting and there is a danger of ignition due to violent heat generation when lithium touches even a slight amount of lithium, so even if it is used as a primary battery that does not charge, secondary chargeable secondary Application to batteries did not spread. Maxell'sCoin type manganese dioxide lithium secondary battery "ML"Although there are lithium batteries that can be recharged like these, these are made mainly by a ceramic separator so that dendrite does not short-circuit the positive electrode and the negative electrode.
Professor Linden Archer who teaches chemistry and biomolecular engineering at Cornell University and a team led by graduate student Snnahasse Chodory have devised ways to suppress the growth of dendrite between the positive and negative poles. What is used is not a ceramic separator but a polymer membrane made with polyethylene oxide crosslinked with poly (propylene oxide) and silicon dioxide. The membrane is perforated, and ions do not pass through the dendrite even though it passes through it. Using the membrane produced this time, it seems that it becomes possible to maintain good conductivity even at room temperature.
The wonderful thing about this "discovery" is that there is no need to fundamentally change the battery technology. As a lithium ion battery also uses a polymer membrane as a separator, the membrane produced this time can be incorporated into various batteries. Furthermore, it may be possible to use other metals that are limited in application by dendrite like lithium and lithium like sodium and aluminum.
Related Posts:
in Science, Posted by logc_nt