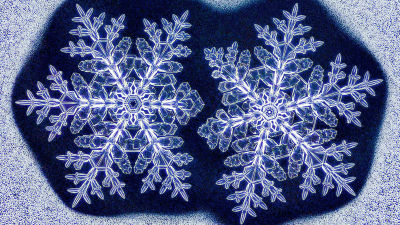
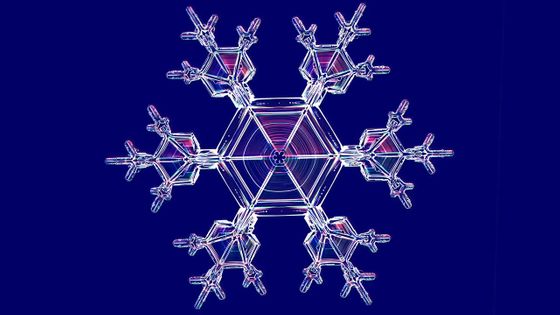
What is the law discovered by a scientist who can freely create snowflakes?
Professor
The Snowflake Myth - YouTube
Libbrecht is a snowflake expert and served as a snowflake consultant on the film ' Frozen .'

'It's fine to make snowflakes with your fingertips, but if they're not real, people won't believe them,' Libbrecht jokes.

The snowflakes that Libbrecht created have been featured on postage stamps, and he has also published many books about snowflakes.

'I call these designer snowflakes,' Libbrecht says of his artificially created snowflakes. 'I created them on the fly, and I don't have a computer to run it. I do it all by hand, so each one is a little different.'

Libbrecht has a very advanced knowledge of snow crystals, so he can design and construct crystal shapes to suit your needs.
This is the crystal that becomes snow. To make it grow branches, you need to lower the temperature to between -13 and -15 degrees Celsius, then raise the temperature again.

Libbrecht manually controls the temperature control knob.

Then, a branch sprouted from the simple hexagonal crystal.

Next, we will raise the temperature to 0 degrees to generate water droplets and make further adjustments.

The snowflakes were created exactly as Libbrecht had designed them.

The snow crystals Libbrecht created artificially are incredibly sharp and clear, because while snow crystals in the wild more or less evaporate before they can be observed, those created in the lab remain intact.
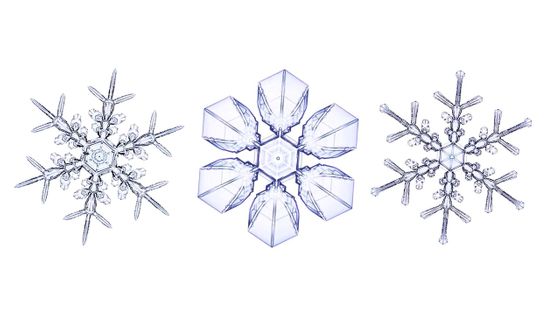
Snowflakes have many characteristics. First of all, all snowflakes are different and no two are the same.
And all the snowflakes grow symmetrically around the hexagon, with the opposite branches having the same shape.

Snowflakes are generally only a few millimeters in diameter, but are also characterized by their extremely thin thickness, measured in micrometers.

Also, while this is the shape that most people associate with 'snowflakes,' there are actually a wide variety of shapes.

These are all snowflakes.

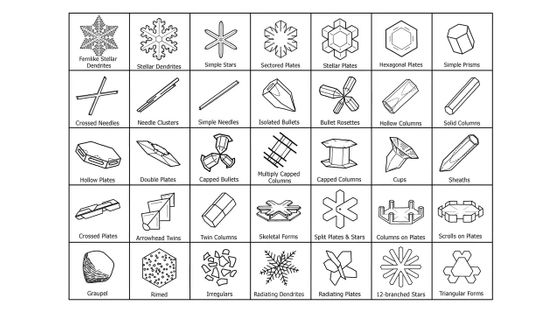
Libbrecht also created a table categorizing snow crystals by type. He said there is no fixed definition of snow, and other researchers categorize snow crystals however they like.
All snowflakes form in a similar way: First, water evaporates into water vapor, and individual molecules fly through the atmosphere. As the water vapor rises, it cools and becomes supersaturated, condensing into tiny droplets on particles like dust.

Even when the temperature is below freezing, water droplets don't freeze immediately, but at some point a droplet will turn into ice, where the water molecules lock together and form hexagonal crystals through hydrogen bonds.
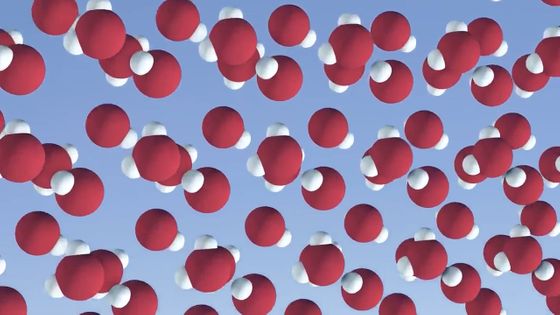
When water molecules gather in a crystal, they form a '
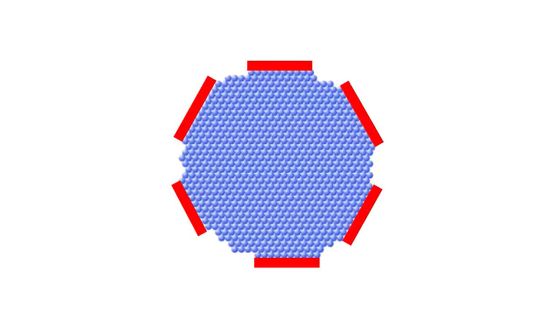
The initial snowflakes are shaped like hexagonal prisms with flat top and bottom surfaces (basal surfaces).

Rapid growth at the basal surface causes the snow crystal to become columnar.

Rapid growth on the sides creates flat snowflakes.

The horns of the snowflake tend to attract water molecules, forming branches. The horns at the end of the branches also attract water molecules, eventually growing into beautiful snowflakes. It takes 100,000 droplets to create a single snowflake, and the process usually takes 30 to 45 minutes.

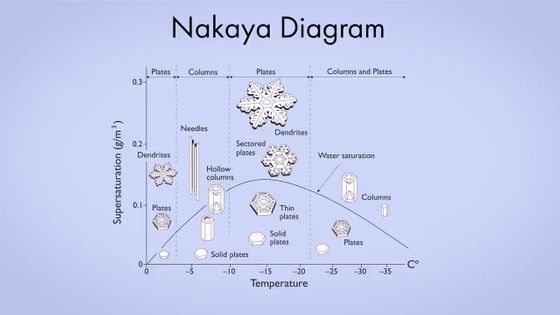
In the 1930s, Japanese researcher

As shown below, the shape of snowflakes formed at different temperatures varies.
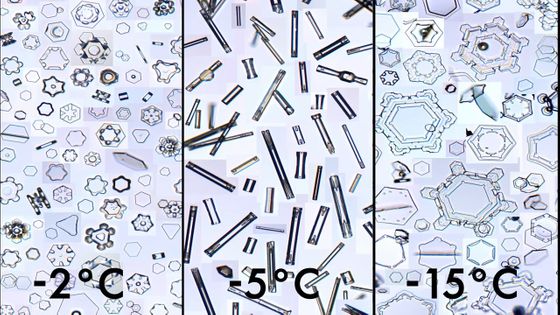
This law is summarized in
Libbrecht says that by looking at the shape of a snowflake, you can understand to some extent 'what kind of environment this crystal grew in.'

The shape of a snowflake depends on various factors, such as the temperature during growth. However, these factors rarely differ between the two ends of a branch growing from a single crystal, so both ends of the crystal grow in the same way. However, because each individual snowflake's environment and trajectory are different, no two snowflakes are the same shape.
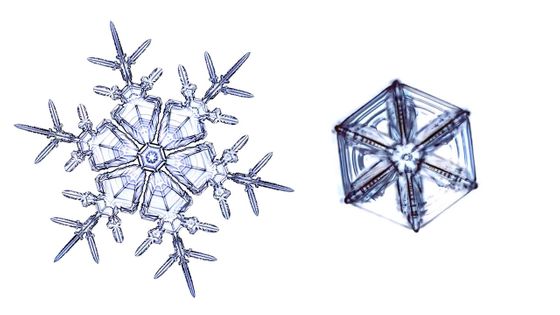
In the lab, the conditions under which snowflakes grow can be precisely controlled, so it's theoretically possible to create identical snowflakes. In Libbrecht's lab, he creates snowflakes using tiny sapphire crystals as nuclei.

Libbrecht actually created identical snowflakes, which he called 'identical twin snowflakes.'

Libbrecht also found an answer to the long-standing question of why snow crystals take the shape shown in the Nakaya diagram. Libbrecht's theory suggests that the nucleation barrier is related to

In a hexagonal crystal, the nucleation barriers on the basal and lateral faces are different. If the nucleation barrier on the lateral faces is low, the crystal will grow planarly. On the other hand, if the nucleation barrier on the basal faces is low, the crystal will grow columnarly.

The nucleation barrier of ice is known to be a function of temperature. Furthermore, Libbrecht hypothesized that the 'area of the crystal' is also involved in the nucleation barrier, and created the following graph. The blue line shows the change in the nucleation barrier for the area of the crystal, and the green line shows the change in the nucleation barrier for the area of the crystal, with respect to temperature.
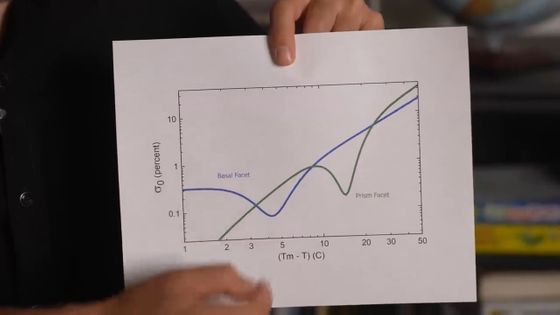
This graph is said to match the shape of a snowflake proposed in the Nakaya diagram.
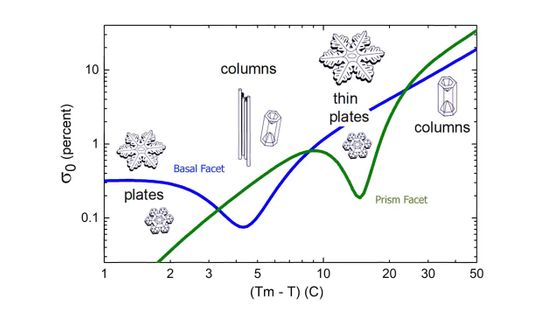
Libbrecht says he conducted experiments and confirmed that this graph is correct.

Related Posts: