Artificial diamonds are superior to natural diamonds in terms of price, beauty, and fewer impurities.

Natural
Lab-grown diamonds - Works in Progress
https://worksinprogress.co/issue/lab-grown-diamonds/
Diamonds are minerals composed only of carbon atoms, and because the bonds between the atoms are very strong and dense, they have the highest Mohs hardness of any natural material, a rating of 10. In addition to giving them an excellent shine as gemstones when certain impurities are present, diamonds are also valuable for industrial purposes due to their low compressibility, high thermal conductivity, and the ability to become a semiconductor when combined with small amounts of nitrogen, phosphorus, or boron.
It is said that it takes billions of years for diamonds to form in nature, and most natural diamonds have too many impurities to be used in jewelry or the high-tech industry, and are expensive to mine. On the other hand, with the development of science and technology in recent years, the manufacturing technology for artificial diamonds has improved, and at the time of writing, they are said to surpass natural diamonds in terms of quality and cost.
'Artificial diamonds are proof of the principle that anything that nature can do, humans can do better,' said Javed Larker , a computer scientist at Harvard University Graduate School, explaining the history of artificial diamonds.

First, in 1796, British chemist
For a long time, the mechanism by which diamonds are formed remained a mystery, but in the late 19th century, Nobel Prize-winning French chemist Henri Moissan began working on creating artificial diamonds. Moissan conducted geological analyses of diamonds in southern Africa, where diamonds are found, and the Canyon Diablo meteorite, which contains moissanite , a mineral similar to diamonds.
Since the diamonds in southern Africa are found in layers deep underground, Moissan speculated that they were formed deep underground under high pressure. Also, since iron has been found in the same geological strata and meteorites where diamonds and similar minerals are found, he speculated that diamonds were formed when hot iron containing carbon was suddenly cooled and violently compressed.
In the end, Moissan himself did not succeed in producing artificial diamonds, but in the 1950s, General Electric in the United States succeeded in synthesizing diamonds for the first time using a belt press type anvil that creates high temperature and high pressure conditions. Tracy Hall , who led the project team, was not treated well at the company and later left to become a professor at Brigham Young University.
The synthetic diamonds produced by Hall's process were tiny, measuring just a few thousandths of a millimeter in diameter, making them unsuitable for jewelry, but were used for precision grinding wheels and saws. General Electric's synthetic diamonds were more expensive than natural diamonds, but they were superior for industrial use because they could be customized to their shape and regularity.

Since then, research into the production of artificial diamonds has been progressing in various countries, and at the time of writing, artificial diamonds of sufficient quality for use as jewelry are being industrially produced. In their pure form, artificial diamonds and natural diamonds are physically, chemically, and optically identical, and it is impossible to tell with the naked eye whether the excellent diamonds on the market are artificial or natural.
A trained jeweler can tell if a diamond is synthetic by looking for impurities and growth patterns during its formation, but synthetic diamonds are now much cheaper than natural diamonds, have higher purity, can be tailored to the consumer's needs for clarity and color, and are easier to cut.
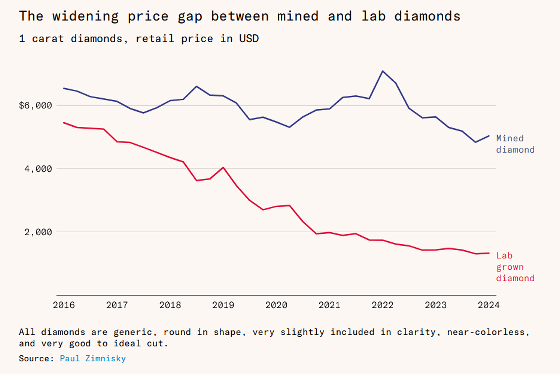
The graph below compares the price trends per carat between natural diamonds (blue) and artificial diamonds (red), with the vertical axis showing price (dollars) and the horizontal axis showing years. As of 2016, natural diamonds were about $6,500 (about ¥930,000) per carat and artificial diamonds were about $5,500 (about ¥790,000) per carat, but the gap is gradually widening, and by 2024, natural diamonds will be about $5,000 (about ¥720,000) and artificial diamonds about $1,300 (about ¥190,000). It seems that sales of artificial diamonds have already surpassed those of natural diamonds.
'Lab-grown diamonds can be fashioned more beautifully than mined diamonds, so we expect their use in fine jewelry to increase,' Larcker said. 'Lab-grown diamonds won't eliminate conspicuous consumption , but most of the benefits will accrue to consumers rather than mine owners.'
◆ Forum is currently open
A forum related to this article has been set up on the official GIGAZINE Discord server . Anyone can post freely, so please feel free to comment! If you do not have a Discord account, please refer to the account creation procedure article to create an account!
• Discord | 'Which is more attractive, artificial diamonds or natural diamonds?' | GIGAZINE
https://discord.com/channels/1037961069903216680/1282998188499927081
Related Posts:
in Science, , Posted by log1h_ik







