How do high-performance masks that protect the human body from toxic gases work?

Gas masks worn by military and terrorists in movies are used to protect people from polluted air and toxic gases. In the same way, ordinary masks with advanced filters can also block toxic gases, but how such masks work is explained in an animation by the YouTube channel TED-Ed.
Gas masks are often seen in movies and TV dramas as intimidating equipment worn by military and combatants, but there are also inexpensive, high-performance masks that have similar functions to gas masks. If toxic gases spread due to forest fires or harmful ozone increases in the atmosphere due to global warming, high-performance masks can protect you from smoke and toxic gases.

The first rule that applies to gas masks and regular masks is that they must be airtight. No matter how good a mask is, it's useless if there are gaps that let air in.

Assuming the mask is airtight, there are two main ways it keeps out contaminants: either it has tiny filters that block particles based on their size, or it attracts and retains certain compounds in some way.
However, at the scene of a forest fire, for example, various chemicals are produced. Firefighters are confronted with high concentrations of these substances at close range, and no filter can block the harmful substances. For this reason, firefighters and others use air supply devices such as oxygen tanks in combination with gas masks.
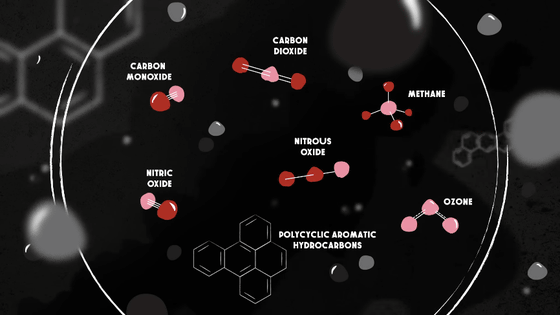
Meanwhile, at a distance, the various chemicals are condensing into tiny solid and liquid particles less than 2.5 microns in diameter -- particles that are dangerous but often visible as smoke, and large enough to be blocked by even the most basic filters.
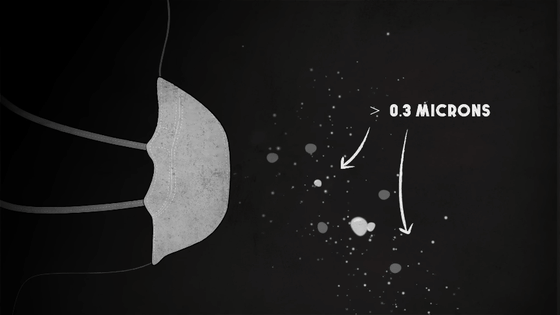
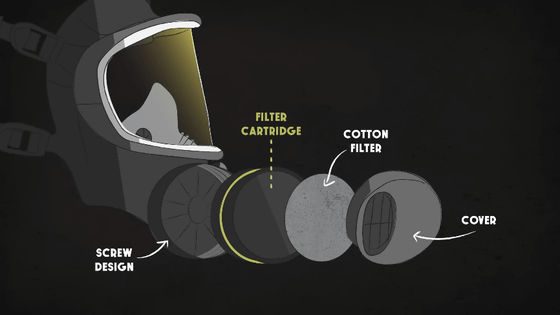
Made from polypropylene or fiberglass, which is roughly one-tenth the width of a human hair, the filters essentially act as a sieve to keep out larger particles.
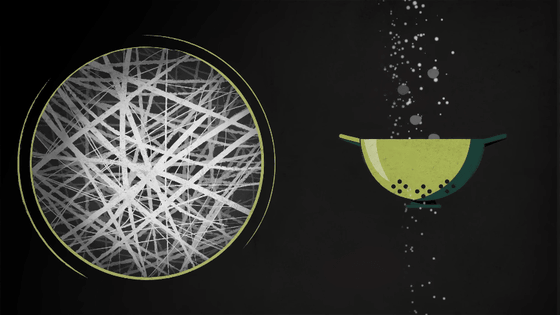
Additionally, polypropylene fibers are able to capture particles much smaller than a fibrous mesh, due to

Furthermore, by using electrically charged fibers within the fabric, it is possible to attract particles that were not on a trajectory that would stick to the fabric.
In this way, a simple 'N95 mask' without any complex features can capture at least 95% of particulate matter through its seal and filter.

In addition, masks that meet the highest standard set by the National Institute for Occupational Safety and Health (NIOSH) in the United States, as well as high-precision air purifiers, are said to be able to capture at least 99.97% of particles.

It is difficult to completely capture harmful substances that are slightly larger than oxygen by sealing or filtering them. For this reason, activated carbon is used as a filter to filter out such substances and allow only safe air to pass through.
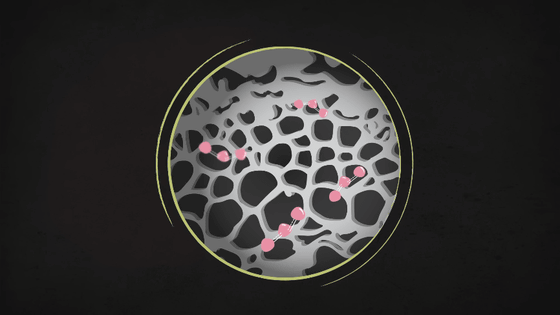
When viewed at a microscopic level, activated carbon looks like a black honeycomb with many holes, and its highly microscopic porous structure allows it to capture small substances such as ozone molecules.
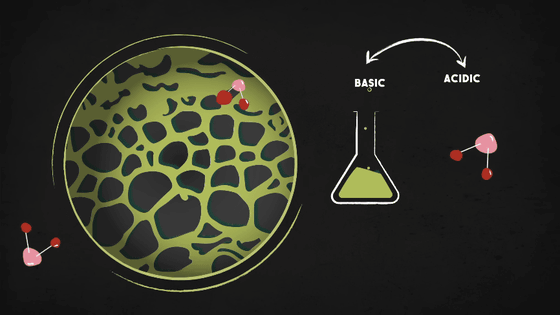
However, activated carbon alone is not enough to block other contaminants like hydrogen sulfide, chlorine, ammonia, etc. Therefore, more advanced protection can be applied through chemical reactions, such as injecting the filter with a basic chemical to trap the contaminants if they are acidic, or injecting an acid to trap basic contaminants.
Gas masks and N100 masks offer a high level of protection through filters and chemical reactions, but they often do not provide complete protection, so if the threat level is high, it is wise to stay indoors, tightly sealed and with the ventilation fans turned off.

Related Posts:







