Introducing a blood sugar monitoring device that can be purchased without a prescription
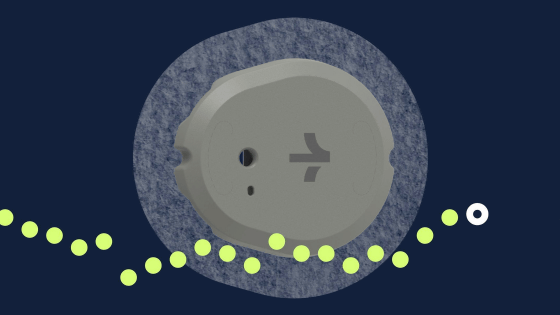
The U.S. Food and Drug Administration (FDA) has granted permission to sell Dexcom's glucose monitoring device ' Stelo ' without a prescription. Stelo is scheduled to be released in summer 2024.
Introducing Stelo, the first FDA-cleared glucose biosensor available without prescription | Dexcom
FDA Clears First Over-the-Counter Continuous Glucose Monitor | FDA
https://www.fda.gov/news-events/press-announcements/fda-clears-first-over-counter-continuous-glucose-monitor
DexCom, Inc. - Stelo by Dexcom First Glucose Biosensor Cleared by FDA as Over-the-Counter
https://investors.dexcom.com/news/news-details/2024/Stelo-by-Dexcom-First-Glucose-Biosensor-to-be-Cleared-by-FDA-as-Over-the-Counter/default. aspx
Diabetic patients need to regularly measure and manage their blood sugar levels. Among the many blood sugar level measurement methods that exist, 'CGM' is a popular method as a blood sugar level visualization system that allows continuous monitoring of blood sugar levels 24 hours a day by attaching a patch with a needle to the body.
Stelo is a CGM developed by Dexcom that can be attached to the back of the upper arm to measure blood sugar levels and view the measurement results on a smartphone app. Continuous operation time is up to 15 days.
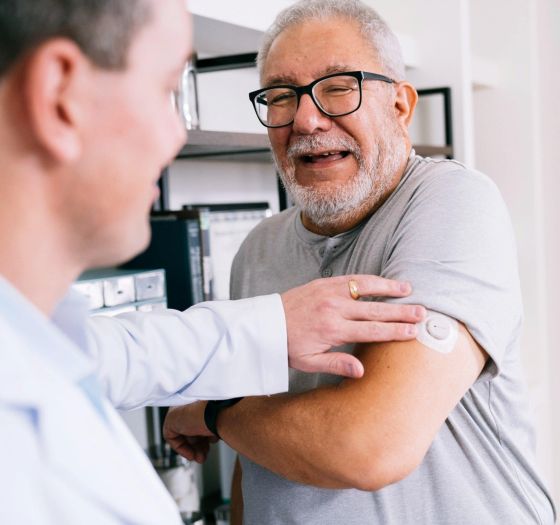
Stelo is scheduled to be released in the United States in summer 2024. While existing CGMs require a doctor's prescription to obtain, Stelo has received 'non-prescription clearance' from the FDA, allowing users to purchase directly online. However, the FDA warns that ``users should not make medical decisions based solely on Stelo measurement results without consulting their health care provider.''
As mentioned above, Stelo is a type of blood sugar measuring device that measures blood sugar levels by sticking a needle into your arm. The FDA has taken a cautious stance toward non-invasive blood glucose monitoring devices that do not use needles, and on February 21, 2024, stated that ``Smart watches and smart rings should not be used to measure blood sugar levels.'' ” issued a statement.
Regulatory authority FDA warns ``Do not use the non-invasive blood sugar measurement function of smart watches'' regarding the blood sugar level measurement function that Apple Watch also aims for - GIGAZINE

◆Forum now open
A forum related to this article has been set up on the GIGAZINE official Discord server . Anyone can write freely, so please feel free to comment! If you do not have a Discord account, please create one by referring to the article explaining how to create an account!
• Discord | “How much would you like a blood sugar monitoring device?” Is it OK to use the needle prick type? ' | GIGAZINE
https://discord.com/channels/1037961069903216680/1215233973542981682
Related Posts: