An innovative treatment for diabetes that produces insulin using bacteria resident on the skin and constantly supplies it to the body is becoming a reality.
If
Getting Under the Skin
https://www.growbyginkgo.com/2024/01/09/getting-under-the-skin/

In 2010, a research team led by molecular biologist Craig Venter actually synthesized DNA containing the genome based on bacterial genome information recorded on a computer and transplanted it into yeast, creating 'synthetic life.' We succeeded in creating this for the first time in the world.
Albert Hayek, professor emeritus of pediatrics at the University of California, San Diego, was so shocked when he read Venter et al.'s paper that he came up with the idea, ``Could this be used to create bacteria that produce insulin?'' About.
Insulin is a hormone produced by tissues within the pancreas, but if for some reason this hormone is no longer produced in the body, the blood sugar level cannot be lowered, leading to diabetes. Type 1 diabetics, in particular, are born with a deficiency in insulin secretion and therefore require regular insulin injections.
However, in the United States, the price of insulin is rising for various reasons, putting pressure on the cost of living for people with diabetes.
The reason why the insulin market is turning into hell in the US is the collusion between pharmaceutical companies and regulatory authorities - GIGAZINE
In 2011, the year after Mr. Hayek read his paper, the J. Craig Venter Institute, run by Mr. Venter, was built near Hayek's home. Mr. Hayek contacted the research institute and became acquainted with John Glass, who heads the synthetic biology group at the Venter Institute. Initially, they planned to conduct research using stem cells, but when they consulted immunologist Richard Gallo, he suggested the idea of using resident bacteria on the surface of the skin.
In 2013, Mr. Gallo published research results showing that bacteria that normally reside on the skin live not only in the epidermis, but also in the body 2 mm deeper than the epidermis, and that some of them may interact with blood vessels. In other words, by modifying the DNA of the resident bacteria in the epidermis and programming it to secrete insulin when it senses a rise in blood sugar levels, it is possible to introduce a system into the body that automatically secretes insulin.
The research team led by Hayek and Gallo modified the DNA of Staphylococcus epidermidis, a type of bacteria that normally lives on the skin, and incorporated a gene that expresses an insulin analogue consisting of a single amino acid chain. This insulin analog works similarly to insulin produced by the body, but is unique in that it is stable at temperatures suitable for S. epidermidis to thrive.
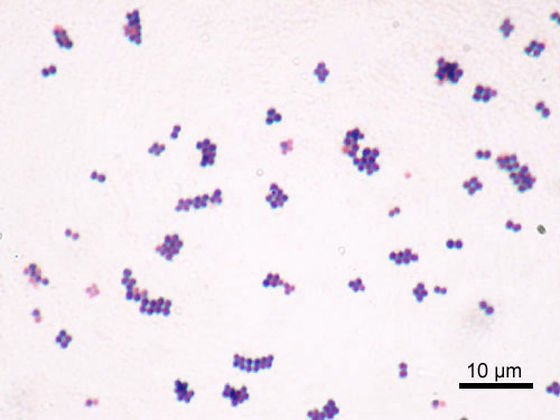
In addition, it is necessary to have a failsafe in case something goes wrong with Staphylococcus epidermidis or if it infects others. Therefore, the research team said that they eliminated from the DNA of Staphylococcus epidermidis the gene necessary to synthesize thymidine, a type of nucleoside that makes up DNA. Therefore, the modified Staphylococcus epidermidis cannot survive unless thymidine is administered externally.
What is even more important is to introduce a mechanism that secretes insulin when needed. Research team member Kaisha Benjamin, a bioengineer at Stanford University, identified several genes whose mRNA levels increase in response to glucose concentrations. Next, they are looking for promoter regions related to the glucose sensing mechanism. Placing this promoter region upstream of the insulin-expressing gene added to the S. epidermidis genome ensures that the bacterium pumps out the appropriate amount of insulin only when blood sugar levels are too high.
This research project has been ongoing for 10 years since 2013, but many issues remain at the time of writing. One is that your body produces less insulin than expected. It is also unclear whether insulin produced from genetically engineered bacteria will work for everyone.
Furthermore, even if all the objectives are achieved, a treatment using resident skin bacteria must be approved by regulatory authorities before it can be put into practical use, and it will take time to achieve that goal.・It costs an enormous amount of labor and budget. However, if the treatment becomes widely available and competition among pharmaceutical companies intensifies, costs could be reduced.
Related Posts:
in Science, Posted by log1i_yk