What is the mechanism by which the new corona drug 'Mornupiravir' works?
On October 1, 2021, pharmaceutical company Merck found in a
How antiviral pill molnupiravir shot ahead in the COVID drug hunt
https://www.nature.com/articles/d41586-021-02783-1
Molnupiravir is a non-profit organization DRIVE (Drug Innovation Ventures at Emory) owned by Emory University in the United States, and was developed as a treatment forVenezuelan encephalitis. And in 2015, Mark Denison, a virologist at Vanderbild University , discovered that molnupiravir was effective against two viruses in the coronaviridae family, the Middle East respiratory syndrome virus and the murine hepatitis virus.
After the outbreak of the new coronavirus pandemic, DRIVE CEO George Painter and Georgia State University virologist Richard Plemper used ferrets to study the efficacy of the new coronavirus against the new coronavirus. It is reported that it was confirmed that replication and infection were suppressed. According to Mr. Premper, molnupiravir is effective against RNA viruses such as the coronaviridae family, similar to remdesivir , which is a treatment for Ebola hemorrhagic fever.
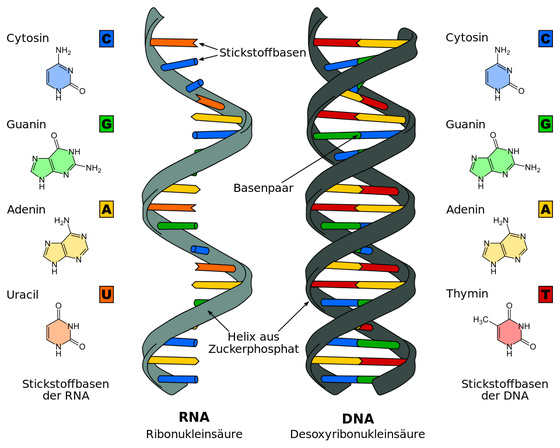
RNA (ribonucleic acid) is a substance in which ribonucleoside, which is composed of four types of bases , adenine , guanine , cytosine, and uracil, and a sugar called ribose , is linked by a phosphate group. It is similar to the DNA (deoxyribonucleic acid) found in human cells, but the types of bases are partially different and the molecular structure is also slightly different.
by
DNA stores genetic information by base sequence, but not all base sequences of DNA are used as information as they are. In the case of humans, the genetic information is transcribed into RNA based on DNA, and the protein is translated from the edited RNA. This edited RNA is called messenger RNA (mRNA).
New coronaviruses and Ebola viruses are classified as RNA viruses that store genetic information directly in RNA rather than in DNA. RNA virus infects cells, sends its own RNA into the cell to read it, and propagates by replicating the proteins and RNA that make up the virus.
Both molnupiravir and remdesivir are called 'nucleoside analogs' and are substances with a structure similar to the ribonucleosides that make up this RNA. Remdesivir blocks viral RNA replication by inhibiting the action of RNA polymerase , an enzyme required to connect ribonucleosides into RNA 'strands.'
Molnupiravir, on the other hand, pretends to be a nucleoside and is incorporated into RNA, which has the function of messing up that RNA. Simply put, Molnupiravir's strategy is to kill the virus by causing an error in the genetic information of the virus.

'When molnupiravir is inserted and a structural change in RNA occurs, mutations occur. When a sufficient number of mutations are accumulated, the viral population collapses. We call this' lethal. ' It is called 'mutation induction'. The virus basically mutates itself and destroys it. ' One of the strengths of molnupiravir is that it is difficult for the virus to develop resistance to molnupiravir because the mutations caused by molnupiravir are random.
However, there are concerns that molnupiravir is mutagenic in human cells, that is, mutations in RNA may be integrated into human DNA, and some researchers have questioned its safety. Merck has not released detailed data on the safety gained in clinical trials, but Dalia Hazda, Merck's Vice President and Chief Scientific Officer for Infectious Disease Drug Discovery, said, 'Use as intended. I'm confident that Molnupiravir is safe, 'he said. On October 11, 2021, Merck applied to the US Food and Drug Administration for an emergency use authorization for molnupiravir.
Merck & Co. Apply for Emergency Use Authorization for Oral Corona | Reuters
https://jp.reuters.com/article/health-coronavirus-merck-co-idJPKBN2H11MR
If molnupiravir becomes a cheap and effective oral remedy, it will be very reliable for human beings to fight the new coronavirus, but at the time of writing it is unclear whether molnupiravir will be available to everyone. The US government has signed a contract with Merck to purchase 1.7 million cases of molnupiravir for 1.2 billion dollars (about 130 billion yen), and from this it can be said that one case of molnupiravir is about 7,000 yen. It's a lot cheaper than Remdesivir, which costs more than $ 2,300 per patient, but I'm not sure if you can get Molnupiravir at a reasonable price even in low- and middle-income countries.
Rachel Cohen, a crew member of the North American Chapter of the Drugs for Neglected Diseases initiative , said, 'Even if poor countries can buy drugs, they may not have the diagnostic ability to properly prescribe molnupiravir. Hmm. In order to administer molnupiravir within 5 days of the onset of symptoms, it is a prerequisite that the doctor actually makes a quick diagnosis. This is a big challenge not only in developing countries but also in wealthy countries. ' I commented.
Related Posts:
in Science, Posted by log1i_yk