LOY-001, an anti-aging drug for dogs that is expected to extend the lifespan of large dogs, takes a step toward approval by the U.S. Food and Drug Administration

In general, the larger the mammal, the longer it lives, but this rule does not apply to dogs, and large dogs such as
FDA Center for Veterinary Medicine agrees Loyal's data supports reasonable expectation of effectiveness for large dog lifespan extension
https://loyalfordogs.com/posts/loyal-announces-historic-fda-milestone-for-large-dog-lifespan-extension-drug

FDA Agreees Loyal Data Supports Reasonable Expectation of Effectiveness for Large Dog Lifespan Extension | Business Wire
https://www.businesswire.com/news/home/20231127326868/en/FDA-Agrees-Loyal-Data-Supports-Reasonable-Expectation-of-Effectiveness-for-Large-Dog-Lifespan-Extension
Anti-aging drug for dogs moves closer to gaining FDA approval
https://www.foxnews.com/health/anti-aging-drug-dogs-san-francisco-firm-moves-closer-gaining-fda-approval
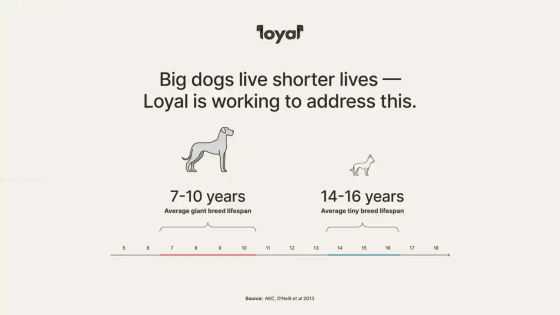
Dogs are animals whose lifespans vary widely depending on their breed; small dogs such as Chihuahuas have an average lifespan of 14 to 16 years, while large dogs such as Great Danes have an average lifespan of 7 to 10 years. .
A major factor that shortens the lifespan of large dogs is the growth hormone
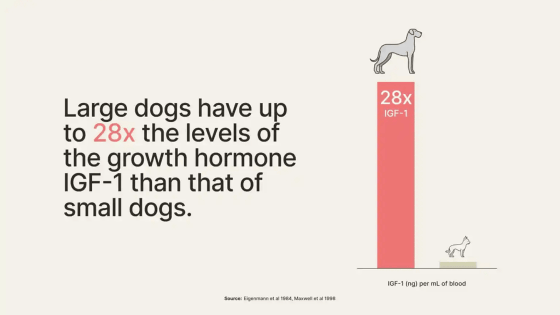
LOY-001, a drug developed by Loyal, reduces IGF-1 in adult dogs and is expected to extend the lifespan of large dogs when administered by veterinarians every 3 to 6 months. . Celine Haoria, Founder and CEO of Loyal, said: 'There are 25 million large breed dogs in the United States alone. This means that anti-aging drugs could help 25 million large breed dogs live longer and improve their quality of life. 'You can do that.'
Loyal has been developing and demonstrating the efficacy of LOY-001 for four years, and has previously conducted research on an FDA-approved canine aging model and an observational study of 451 dogs. He said. In November 2023, the FDA finally confirmed the drug's 'efficacy' in order to grant 'conditional approval' for the use of LOY-001.
This means that the FDA has found that LOY-001 has a reasonable expectation of efficacy. In the future, if the FDA approves the manufacturing and safety data package for LOY-001, Loyal will be able to market LOY-001 under conditional approval to extend the lifespan of eligible dogs. The conditional approval will be in effect for up to five years, during which time Loyal plans to collect remaining efficacy data and apply for full approval based on that.
Dr. Ivana Krunec, a veterinarian not affiliated with Loyal, told Fox News: 'In my professional opinion, this drug is a breakthrough. We will have to wait and see the results and potential side effects. , LOY-001 is definitely promising so far, and the fact that the FDA has determined that this drug has a 'reasonable expectation of efficacy' speaks volumes about its potential.' .

Related Posts:







