Amazon begins development of cancer vaccine, joint research with cancer research facility

Amazon is reportedly developing a cancer vaccine in collaboration with the Fred Hutchinson Cancer Research Center in Seattle, Washington, and plans to begin FDA-approved clinical trials.
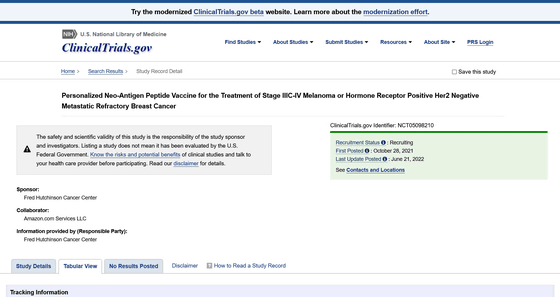
Personalized Neo-Antigen Peptide Vaccine for the Treatment of Stage IIIC-IV Melanoma or Hormone Receptor Positive Her2 Negative Metastatic Refractory Breast Cancer --Tabular View --ClinicalTrials.gov
https://clinicaltrials.gov/ct2/show/record/NCT05098210
Amazon partners with Fred Hutchinson on cancer vaccine trial
https://www.cnbc.com/2022/07/12/amazon-partners-with-fred-hutchinson-on-cancer-vaccine-trial.html
Amazon and Fred Hutchinson Cancer Research Center are recruiting 20 subjects over the age of 18 for Phase I clinical trials in an application to clinicaltrials.gov, a clinical trial database run by the National Library of Medicine in the United States. I am.
The purpose of the clinical study was stated in the application form that it was to develop a vaccine to treat melanoma, which is a type of skin cancer, and breast cancer (hormone receptor positive HER2 negative). The clinical trial was filed in October 2021 and is expected to begin June 9, 2022 and be completed by November 1, 2023.
It is reported that this Amazon cancer vaccine development project started from Amazon's secret research project 'Grand Challenge'. As of June 2018, a representative of the Fred Hutchinson Cancer Research Center told CNBC, an economic news media, that 'a project is underway with several tech neighbors.' rice field.
An Amazon spokeswoman has acknowledged the collaboration with the Fred Hutchinson Cancer Research Center and said the research is being led by the Fred Hutchinson Cancer Research Center.
In addition, an Amazon spokeswoman said, 'Amazon is providing science and machine learning expertise to our partner, the Fred Hutchinson Cancer Research Center, seeking to develop personalized treatments for specific cancers. Very early on, the Fred Hutchinson Cancer Research Center has been licensed by the US Food and Drug Administration to proceed with Phase I clinical trials, but it is unclear if it will succeed. This will last for several years. It's a long process, and as it progresses, we can work with other healthcare and life sciences organizations that may be interested in similar efforts. '
Related Posts: