Chronic low back pain treatment device developed that deploys in the spinal column and sticks to the spinal cord

A research team at the University of Cambridge, England, has announced a spinal cord stimulator suitable for the treatment of severe chronic low back pain for which the drug is not expected to be effective. It is reported that this device has the
Electronics with shape actuation for minimally invasive spinal cord stimulation | Science Advances
https://advances.sciencemag.org/content/7/26/eabg7833
Inflatable, shape-changing spinal implants could help treat severe pain
https://www.cam.ac.uk/stories/spinal-implants
Mind-Blowing'Inflatable' Spinal Cord Implant Could Make Pain Relief Widely Available
https://www.sciencealert.com/mind-blowing-spinal-cord-implant-could-relieve-suffering-for-chronic-pain-patients
Spinal cord stimulation is a type of surgical treatment in which weak electricity is applied from a device implanted in the spine to relieve intractable pain. While it is said to be effective for severe low back pain where the drug does not work, there is a risk that electrodes need to be pierced into the human spinal column and placed throughout the spinal cord. There are two types of existing spinal cord stimulation therapy devices, a paddle type that covers the spinal cord and a needle type that pierces the spinal cord. The paddle type has a risk that a part of the vertebra must be removed, and the needle type has a part of the vertebra. Although it does not need to be removed, there is a problem that the effect is low because the contact area between the device and the spinal cord is small.
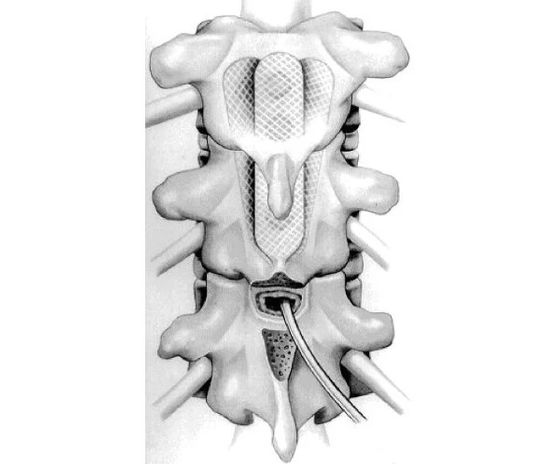
By Krishna Kumar et al
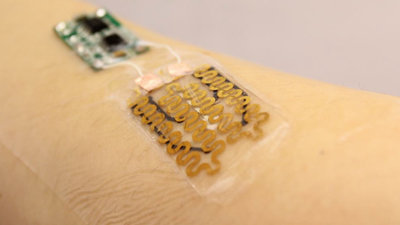
The spinal cord stimulator newly created by Ben Woodington and others at the University of Cambridge has the advantages of the existing paddle type and needle type, and is only 2 mm needle type when stored (left image), but when deployed. The structure is a paddle type with a thickness of 60 microns (right image).
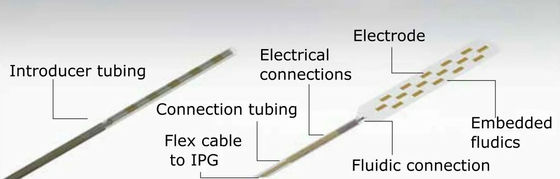
In the needle-type state of the spinal
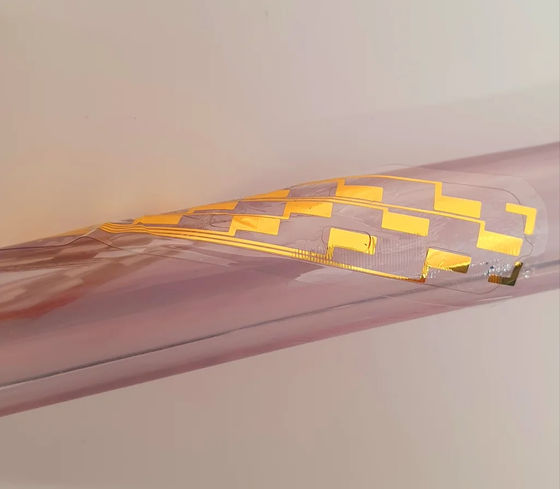
Back pain is a major cause of disability in the United Kingdom, and it is estimated that £ 12 billion is spent annually on treatment. On the other hand, the Centers for Disease Control and Prevention estimates that 1 in 12 people in the United States suffers from intractable back pain for which drug treatments such as non-steroidal anti-inflammatory drugs and opioids do not work.
The spinal cord stimulator developed this time has been tested for electrode functionality in the laboratory and verified by donation, and large-scale tests and clinical trials are required for practical use. 'Our goal was to create a device that combines the best of both worlds, clinically effective, but without the need for complex, high-risk surgery,' said Dr. Christopher Proctor of the research team. Devices may be able to provide more people with life-changing treatments, 'he said. 'The spinal cord stimulator we have developed can be a cure for paralysis due to spinal cord injury and stroke sequelae, and for movement disorders such as Parkinson's disease. It does not require invasive surgery. Effective devices will bring salvation to many people. '
Related Posts: