The mechanism by which lithium-ion batteries deteriorate has been elucidated

Lithium-ion batteries, which are used as power sources for smartphones and electric vehicles, are known to lose capacity over time. A research team from Stanford University has uncovered the mechanism behind this deterioration.
Persistent and partially mobile oxygen vacancies in Li-rich layered oxides | Nature Energy
Scientists discover how oxygen loss in a lithium-ion battery's voltage | SLAC National Accelerator Laboratory
According to the research team, the deterioration of lithium-ion batteries occurs when oxygen contained in the lithium-ion battery leaks out. However, the amount of oxygen leaking out after 500 charging cycles is only 6% of the total, and the amount of oxygen leaking out per charging cycle is too small, so the mechanism has not been observed. Therefore, in this study, the mechanism of oxygen leakage was indirectly observed by observing the chemical properties and structure of the surrounding particles that change due to the loss of oxygen.
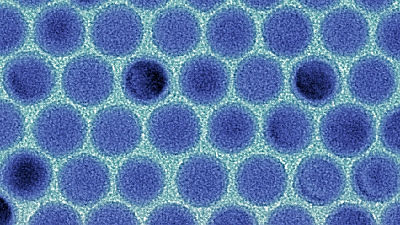
The team disassembled lithium-ion batteries that had gone through various charging cycles and used a specialized X-ray microscope at Lawrence Berkeley National Laboratory to view the nanoparticle structure that makes up lithium-ion batteries on a scale of one billionth of a meter.
According to the research team, it was previously thought that oxygen in lithium-ion batteries leaks out from the surface of nanoparticles. However, the results of this observation showed that oxygen first leaks out from the surface of the nanoparticles, and then leaks out from inside the nanoparticles. In addition, it was also revealed that the amount of oxygen leaking out is reduced when the nanoparticles form agglomerates.
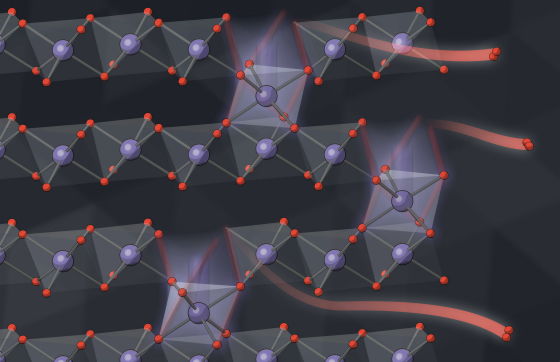
Furthermore, the research team predicted that 'nanoparticles that lose oxygen will collapse inward to form a close-packed structure ,' and analyzed the structural changes of nanoparticles after losing oxygen using computer simulations. Contrary to their prediction, the results showed that 'although the metal ions that make up the nanoparticles move, the structure of the nanoparticles does not change.'
Will Chueh, a member of the research team, expressed his delight at the discovery, saying: 'This rearrangement of metal ions is triggered by a lack of oxygen, which reduces the battery's voltage and efficiency over time. We've known the general outline of this phenomenon for some time, but the mechanism was unknown.'
'The scientific understanding gained from this research could lead to the development of new ways to mitigate oxygen loss and its damaging effects,' says Chue, who is also looking to advance research into reducing the degradation of lithium-ion batteries.
Related Posts:
in Free Member, Science, Posted by log1o_hf