Stories of twists and turns of pharmaceutical companies that won approval of anti-cancer drugs on the verge of collapse
ByDerek K. Miller
Known as cancer of the bloodMalignant lymphomaAs new drugs in 2013American Food and Drug Administration(FDA) approved anticancer drug is "Iburutinib"is. Although approval is continued in European countries as well, although the effect as an anticancer agent is recognized, the pharmaceutical startup which is the developer of ibutinib which continued the development of an anticancer agent drug up to the point of bankruptcyPharmacyclicsThe twists and turns of "are summarized.
Pharmacyclics' 'miracle cure': A cancer drug - San Jose Mercury News
http://www.mercurynews.com/science/ci_29313049/pharmacylics-miracle-cure-cancer-drug
Jonathan Ceciler and Richard Miller, co-founder of Pharmacyclics, met at Stanford University in the 1970s. At that time, Mr. Ceciler is a student who studies hard to earn a bachelor's degree in chemistry, and at the same timeHodgkin's lymphomaWe also fought against malignant lymphoma called. Mr. Miller who cured cancer now is Stanford University's oncologist, cancer drug "RituxanWe have a track record as a businessman supporting IDEC Pharmaceuticals (present: Biogen), a manufacturing company of "
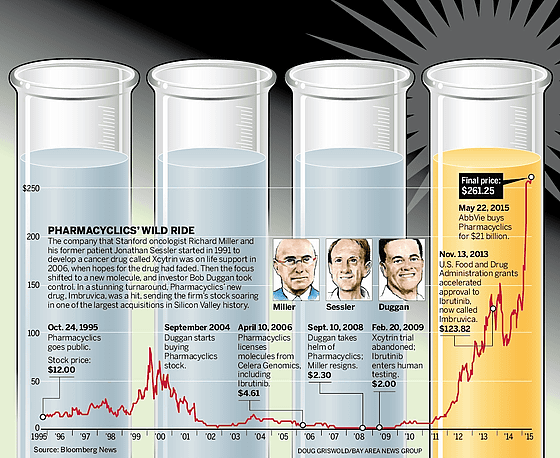
Mr. Miller has supported the fight against cancer for many years as Mr. Cecillar's doctor, and Mr. Ceciler was struggling to study new anticancer drugs while fighting with cancer himself. Mr. Cecilah later continued his research at the University of Texas, and as a result, discovered molecules that could reduce cancer cells and have the potential to suppress formation and metastasis. Based on these studies, Mr. Cecilla and Mr. Miller founded Pharmacyclics in 1991. At that time, the two were betting everything on a new drug called "Xcytrin". This new medicine was expected to have the effect that cancer cells divide the cancer cells into effective size and do not spread to the brain.
The final goal is to be approved by the FDA for new drugs. The way to get approval is long, and if you average all the new drug candidates, it will cost at least 10 years, and it will cost more than $ 2.6 billion (about 310 billion yen) before reaching the patient from the research stage . Pharmacyclics also required a large amount of cash to obtain FDA approval where Mr. Bob Dagan participated as a new investor. Dagan was an excellent investor, but he did not have knowledge of the pharmaceutical industry. However, Mr. Cecillah and Mr. Miller are fighting against cancer, overlapping with the son who died of cancer, and he seems to have received individual support.
However, although Xcytrin showed some extent of life survival against cancer, it was not able to clear the FDA standards and stopped applying for approval at the end of 2007. Pharmacyclics' stock price plummeted to 2 dollars, Cecilah and Miller who are members of the board resign. Like many other bioengineered startups, Pharmacyclics, which has bet on every single product, has been forced into a devastating situation.
Meanwhile, in 2006 before the collapse of Pharmacyclics Mr. Miller heard that a company called Celera Genomics sought to sell molecules at the R & D stage, and discovered some chemical substances among them. Mr. Miller found out the potential as a potential anticancer drug from his medical background and bought Celera's research and development project for each researcher for $ 6 million (about 710 million yen). The chemical substance is a substance having a possibility of inhibiting a protein called "Breton type tyrosine kinase", which was supposed to be effective for treatment of malignant lymphoma, but Mr. Miller said "It was a backup of Xcytrin" It is.
The story returned to 2007, and Mr. Bob Dagan became CEO in Pharmacyclics that the board of directors left. Mr. Dagan himself invested $ 6 million (about 710 million yen) on his own stomach and started a clinical trial on human beings of the Breton type tyrosine kinase that Mr. Miller considered as "backup". As a result of the clinical trial, it was possible to prove that the new drug has an effect on the cancer cell, although some normal cells were also damaged, it was small as toxicity and was within the control range.
It is FDA that has long stood before Pharmacyclics until then, but in order to promptly approve the Breton type tyrosine kinase, we will introduce a system called "breakthrough therapy" to promote the approval of a new drug. And in 2013, the Breton type tyrosine kinase was approved as an anticancer drug called "Iburutinib". Iburutinib does not completely remove cancer, but it can prolong survival benefit by taking it every day, it does not affect normal cell proliferation, so it is fundamental to treat conventional anticancer drugs I decided to change it. Today, approval of Iburtinib is advanced in 46 countries, and annual sales are expected to reach 5 billion dollars (about 600 billion yen).
Related Posts:
in Note, Posted by darkhorse_log