A movie explaining what "fire" is scientifically "What Is Fire?"

A movie that scientifically explained the question about fire, such as "Why is the fire burning?" And "What is the chemical reaction occurring in the flame?"What Is Fire?(What is fire?) ".
What Is Fire? - YouTube
I am a British chemistMichael FaradayIn the mid 1800 's, at the Royal Institute in London he gave a lecture for Christmas for children. It seems that it was to talk about "fire" that was Faraday's favorite.

"The fact that Faraday was particularly interested in was the candle of a candle because it was easy to know what kind of reaction the fire caused despite the delicate flame of the candle" Saying, why MuHumaro covered with bonfires.

When expressing "fire" by a chemical formula, it is only expressed as "methane (CH 4) and oxygen (2 O 2) react to change to carbon dioxide (CO 2) and water (2 H 2 0)".
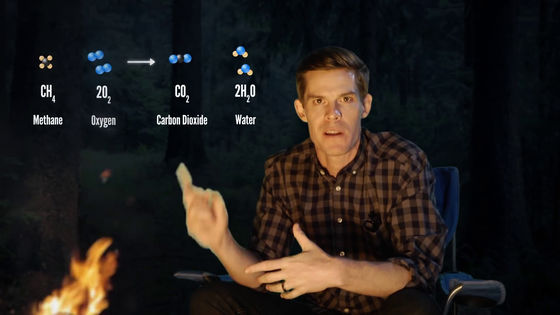
The chemical reaction when the fire burns is actually the same as the process of baking the chocolate chip cookie.

The first thing you need to know about a candle flame is the color of a flame. When a substance emits heatBlackbody radiationThe hot one shines brightly.

For example, the bottom of a candle's flame reaches a high temperature of 1000 to 1400 degrees, so it glows blue.

The center of the flame is 800 to 1000 degrees and somewhat lower in temperature, it has a yellowish orange color.

In fire, chemical reactions are taking place continuously like dozens of fireworks.

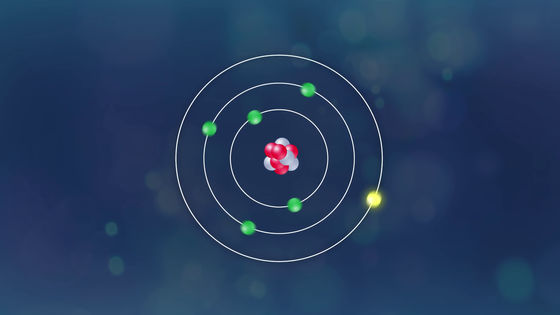
There is no reaction of oxygen in the air and carbon and hydrogen contained in the candle in the elementary state.
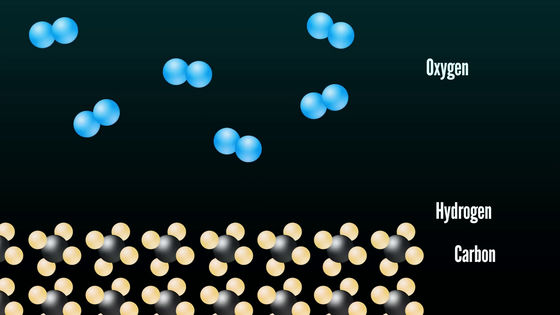
However, when lighting the candle ......

The solid of the wax vaporizes and splits into small chunks, causing thermal decomposition. And the array of atoms changes as the heated hydrocarbon and oxygen collide in the air.


This state is the black cone part around the candle's core.

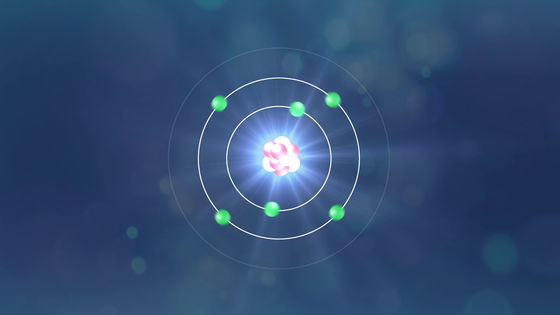
At this time, the array of electrons in the atom changes ... ...
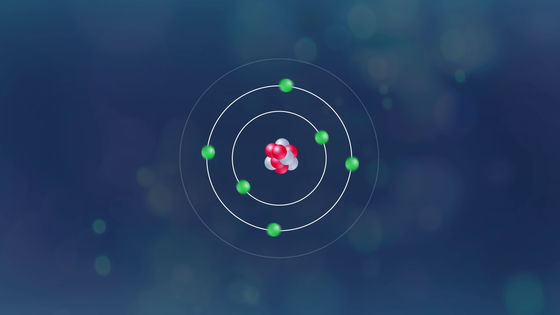
A blue flame at the top of the candle will be born.
Also, since the carbon contained in the candle does not all change to carbon dioxide, the remaining carbon atoms combine and soot is born.
As soot is heated, the top of the candle shines orange.

When all of the soot is burned out, carbon dioxide and water are scattered in the air.

The nature of each part of the candle can be known easily simply by holding the metal product over the candle of the candle. For example, at the top of a candle ...

Water vapor and soot stick to the metal surface.

Hold the spoon over the middle part of the flame ......

A white line is attached to the spoon, and it turns out that vaporized wax is generated.

Next is about the shape of the flame. Cold and dense air is drawn to the ground by gravity, while warm air goes up. In other words, the flame keeps a cone shape due to the buoyancy of air.

If you ignite in a zero gravity space like the space station, the shape of the flame will be different from the ground.

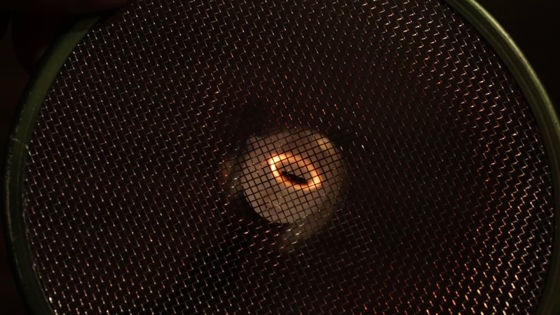
Chemical and quantum reactions occur when fire burns occur only in the presence of air. Therefore, even though the flame appears to be conical, it is actually possible to change the shape. For example, when you try to push a flame from the candle with a wire mesh ......
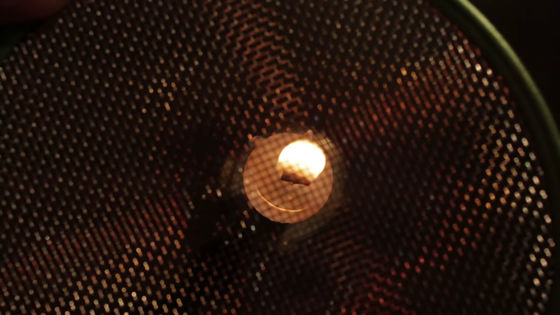
The flame has changed to a shape that collapsed into a peach. The reason why the fire continues to burn is not the division of the molecule, but the bond of the new molecule, and the reaction is sustained by the heat generated when the molecule bonds.
At the end of the movie, bring the fire of the match closer to the candle just turned off ... ...

Even though the fire of the match did not touch the candle's core directly, an experiment was held that the candle glows a fire.

This experiment is a mechanism that vaporized wax rises with smoke immediately after extinguishing the candle's fire, so that the candle can be reignited by smoking.

Related Posts: