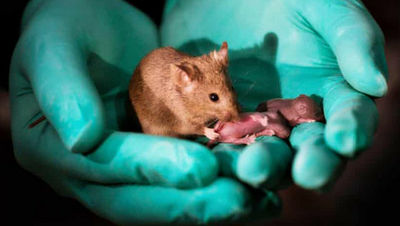
Mice born from male ES cells and sperm survive to adulthood

A research team from the Chinese Academy of Sciences announced that they have succeeded in producing mice from two males using sperm and sperm-derived
Adult bi-paternal offspring generated through direct modification of imprinted genes in mammals: Cell Stem Cell
https://www.cell.com/cell-stem-cell/fulltext/S1934-5909(25)00005-0
Mouse with two fathers survives to adulthood, marking scientific milestone
https://phys.org/news/2025-01-mouse-fathers-survives-adulthood-scientific.html
According to the research team, there have been attempts to produce mice through single-sex reproduction between males, but the embryonic development stops at a certain stage and they rarely grow to adulthood. The research team that announced this time also conducted an experiment to produce mice through single-sex reproduction between males in 2018, but it was reported that the mice born could not survive for more than 48 hours after birth.
Chinese research team succeeds in producing baby mice from two male mice - GIGAZINE
According to the research team, the reason why embryos do not develop during single-male reproduction is due to a gene expression control mechanism unique to mammals called 'imprinting (uniparental expression)'. Normally, our genes are active through both the genes inherited from our father and mother, but with imprinted genes, only one of the genes from the father or mother is expressed, and the other is inactivated. This is because imprinting is a system acquired during the evolution of mammals, and it is said that the father's genes work to promote the growth of the offspring, while the mother's genes work to suppress moderate growth.
For example, the growth-related gene 'IGF2' only works if it is derived from the father, not from the mother. Conversely, the H19 gene only works if it is derived from the mother. Since such gene regulation plays an important role in the normal development of the fetus, it is thought that if only the father's genes are present, the balance between promotion and regulation will be disrupted and development will fail.
In this study, the research team first identified and modified 20 genetic regions related to imprinting in sperm-derived haploid ES cells , and then created bi-paternal embryos by injecting sperm-derived ES cells and sperm into enucleated eggs.
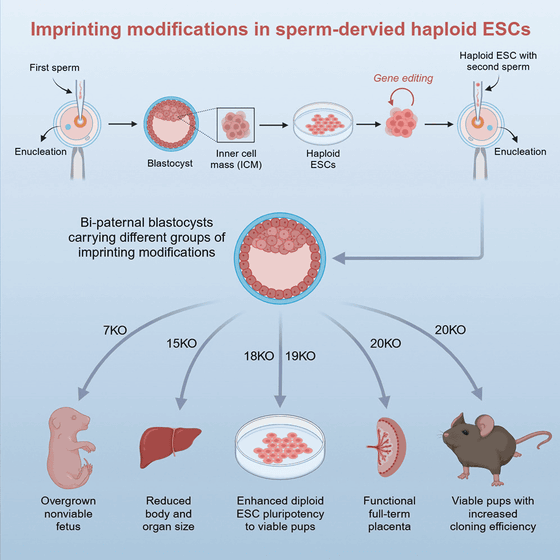
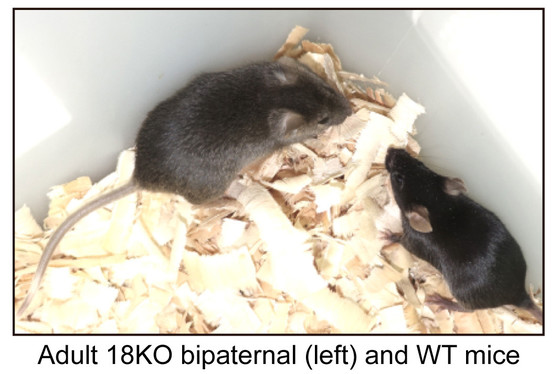
During the course of the experiment, the research team gradually increased the number of gene modifications. In the early experiments in which seven genes were modified, the bi-paternal mice died shortly after birth, but in the experiment in which 18 genes were modified, some of the mice were able to grow to adulthood. Finally, individuals with 20 genes modified were able to form normal placentas and achieved a higher survival rate.
Several characteristic phenotypes were observed in bi-paternal mice that grew to adulthood. For example, bi-paternal mice grew faster, had less anxiety-like behavior, and had reduced hippocampal volume compared to normal mice. In addition, their survival rate under normal rearing conditions without artificial feeding was relatively low at about 36.7%, and their lifespan was about 60% of normal.
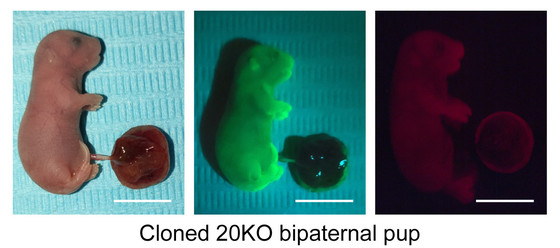
Furthermore, the research team performed nuclear transfer cloning experiments using somatic cells of the bi-paternal mice they created, and succeeded in producing one cloned mouse. This result shows that the modified imprinted gene pattern is stably maintained in somatic cells.
The research team pointed out that the method established in this study may be applicable to improve the maintenance of pluripotency of ES cells and iPS cells, and to improve the efficiency of cloning animals. They also expressed hope that it may lead to the development of treatments for diseases caused by specific imprinting abnormalities. However, they predicted that applying this technology to larger animals would require a considerable amount of time and effort, because the combinations of imprinted genes differ depending on the species.
The research team pointed out that the ethical guidelines of the International Society for Stem Cell Research do not permit the application of this technology to humans from a safety standpoint, and is therefore cautious about putting it to practical use.
Related Posts: