Japanese research team uncovers mechanism by which COVID-19 worsens, may be caused by abnormal adhesion of immune cells
A research group including Hirofumi Ueki, senior researcher at the National Center for Global Health and Medicine, and Yoshihiro Kawaoka, director of the Center for Infectious Diseases at the same center and the International Institute for Advanced Studies at the University of Tokyo, has announced that they have elucidated the mechanism by which novel coronavirus disease (COVID-19) becomes severe.
Neutrophil adhesion to vessel walls impairs pulmonary circulation in COVID-19 pathology | Nature Communications
Elucidating the mechanism by which immune cells cause COVID-19 to worsen | University of Tokyo
https://www.u-tokyo.ac.jp/focus/ja/press/z0406_00022.html
The research team infected seven different disease model mice with a mouse-adapted form of the new coronavirus (SARS-CoV-2) and evaluated their survival rates after infection. As a result, it was found that obese/diabetic model (Ob/Ob) mice had a significantly lower survival rate than wild-type mice.
In addition, two days after infection, large amounts of virus were detected in the lungs and nasal turbinates of both wild-type and Ob/Ob mice, but five days after infection, the amount of virus in the lungs of Ob/Ob mice was significantly higher than that of the wild-type mice.
Micro-CT analysis revealed that the infected Ob/Ob mice had heterogeneous and unclearly defined pneumonia similar to COVID-19 pneumonia. Histopathological analysis also confirmed infiltration of inflammatory cells such as neutrophils and monocytes in the alveolar regions of Ob/Ob mice, and viral antigens were detected in alveolar and bronchiolar epithelial cells. Based on these results, the research team argues that the pathology of infected Ob/Ob mice reflects the pathology of severe COVID-19.
The research team then created a mouse-adapted SARS-CoV-2 strain incorporating a fluorescent reporter gene and visualized infected cells in Ob/Ob mice. Furthermore, by intravenously administering fluorescently labeled dextran and fluorescently labeled antibodies, they were able to visualize pulmonary blood vessels, neutrophils , and platelets . This made it possible to observe dynamic phenomena that could not be captured by conventional analysis.
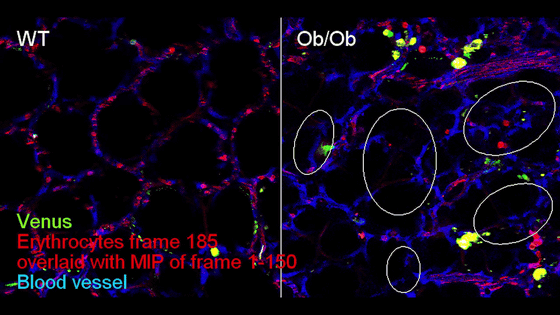
Below, the lungs of a wild-type mouse (left) and an Ob/Ob mouse (right) were observed with a two-photon microscope . Venus (green) visualizes infected cells, Ly-6G (red) visualizes neutrophils, CD41 (blue) visualizes platelets, and Dextran (white) visualizes pulmonary blood vessels.
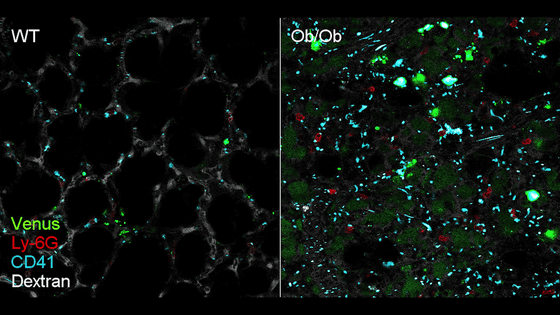
According to the research team, in SARS-CoV-2-infected lungs, the proportion of neutrophils with 'slow movement' speeds of less than 50 µm/s increased, while the proportion of neutrophils with 'fast movement' speeds of more than 50 µm/s decreased.
In addition, the percentage of platelets smaller than 8.57 µm2 decreased, while the percentage of platelet aggregates (8.57-34.28 µm2 ) and thrombi (greater than 34.28 µm2) increased in the infected lungs, suggesting that platelets interact with neutrophils to form microthrombi.
In addition, observations using fluorescently labeled red blood cells confirmed that pulmonary blood flow was significantly inhibited in infected lungs. In the image below, the pulmonary blood flow in a mouse is observed. In a wild-type mouse (left), the vascular structure and the path through which red blood cells (red) flow are almost identical, but in an infected mouse (right), there are areas in many blood vessels (blue) through which red blood cells cannot pass.
Furthermore, the expression of
Below is an image of the blood vessels of an infected Ob/Ob mouse. The yellow triangles indicate aggregated platelets (CD41, blue), the white triangles indicate stagnant red blood cells (Erythrocytes, red), and the white arrows indicate neutrophils (Ly-6G, purple) adhering to the vessel wall. It can be seen that the adhesion of neutrophils to the vessel wall causes platelet aggregation and impaired blood flow.
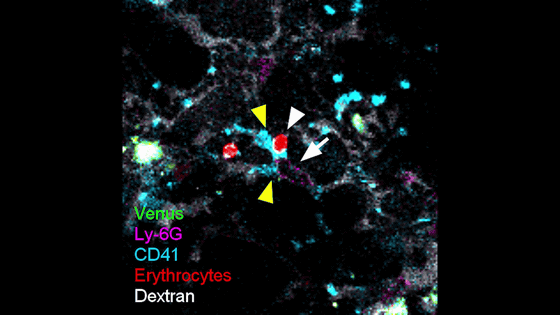
The research team stated that this discovery is consistent with the results of blood sample analysis of severe COVID-19 patients in humans, and pointed out that abnormal adhesion of neutrophils to the vascular wall and subsequent formation of microthrombi by platelet aggregation may inhibit pulmonary blood flow and exacerbate COVID-19 pneumonia. This discovery indicates a new pathological mechanism of SARS-CoV-2 infection that could not be captured by conventional histopathological or histochemical analyses.
However, it is not yet fully understood why adhesion molecules increase upon infection with SARS-CoV-2. The research team suggests that the expression of CD44 may have increased in response to inflammatory cytokines, which are signal molecules secreted by other cells, or that SARS-CoV-2 infection may promote the proliferation of neutrophils in the bone marrow, resulting in the recruitment of large amounts of immature neutrophils. However, further research is needed to fully understand the mechanism behind the increase in adhesion molecules.
The findings of this study on the mechanism of COVID-19 aggravation are expected to lead to the development of new treatments for severe COVID-19 patients and those suffering from sequelae. In particular, the research team stated that therapeutic approaches targeting abnormal neutrophil adhesion and platelet aggregation may provide new options for treating severe COVID-19 patients and those suffering from sequelae.
Related Posts:
in Science, Posted by log1i_yk